Carboxyl-Terminal SSLKG Motif of the Human Cystinosin-LKG Plays an Important Role in Plasma Membrane Sorting
- PMID: 27148969
- PMCID: PMC4858208
- DOI: 10.1371/journal.pone.0154805
Carboxyl-Terminal SSLKG Motif of the Human Cystinosin-LKG Plays an Important Role in Plasma Membrane Sorting
Abstract
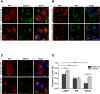
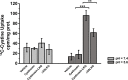
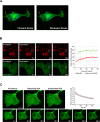
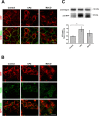
Cystinosin mediates an ATP-dependent cystine efflux from lysosomes and causes, if mutated, nephropathic cystinosis, a rare inherited lysosomal storage disease. Alternative splicing of the last exon of the cystinosin sequence produces the cystinosin-LKG isoform that is characterized by a different C-terminal region causing changes in the subcellular distribution of the protein. We have constructed RFP-tagged proteins and demonstrated by site-directed mutagenesis that the carboxyl-terminal SSLKG sequence of cystinosin-LKG is an important sorting motif that is required for efficient targeting the protein to the plasma membrane, where it can mediate H+ coupled cystine transport. Deletion of the SSLKG sequence reduced cystinosin-LKG expression in the plasma membrane and cystine transport by approximately 30%, and induced significant accumulation of the protein in the Golgi apparatus and in lysosomes. Cystinosin-LKG, unlike the canonical isoform, also moves to the lysosomes by the indirect pathway, after endocytic retrieval from the plasma membrane, mainly by a clathrin-mediated endocytosis. Nevertheless, silencing of AP-2 triggers the clathrin-independent endocytosis, showing the complex adaptability of cystinosin-LKG trafficking.
Conflict of interest statement
Figures




Similar articles
-
Cystinosin-LKG rescues cystine accumulation and decreases apoptosis rate in cystinotic proximal tubular epithelial cells.Pediatr Res. 2017 Jan;81(1-1):113-119. doi: 10.1038/pr.2016.184. Epub 2016 Sep 22. Pediatr Res. 2017. PMID: 27656773
-
Identification and subcellular localization of a new cystinosin isoform.Am J Physiol Renal Physiol. 2008 May;294(5):F1101-8. doi: 10.1152/ajprenal.00413.2007. Epub 2008 Mar 12. Am J Physiol Renal Physiol. 2008. PMID: 18337546
-
Distribution of cystinosin-LKG in human tissues.Histochem Cell Biol. 2012 Aug;138(2):351-63. doi: 10.1007/s00418-012-0958-8. Epub 2012 Apr 29. Histochem Cell Biol. 2012. PMID: 22544350
-
New aspects of the pathogenesis of cystinosis.Pediatr Nephrol. 2003 Mar;18(3):207-15. doi: 10.1007/s00467-003-1077-5. Epub 2003 Feb 27. Pediatr Nephrol. 2003. PMID: 12644911 Review.
-
[From gene to disease: cystinosis].Ned Tijdschr Geneeskd. 2004 Mar 6;148(10):476-8. Ned Tijdschr Geneeskd. 2004. PMID: 15042893 Review. Dutch.
Cited by
-
A Genetic Screen for Investigating the Human Lysosomal CystineTransporter, Cystinosin.Sci Rep. 2018 Feb 21;8(1):3442. doi: 10.1038/s41598-018-21483-x. Sci Rep. 2018. PMID: 29467429 Free PMC article.
-
The gene therapy for corneal pathology with novel nonsense cystinosis mouse lines created by CRISPR Gene Editing.Ocul Surf. 2023 Jul;29:432-443. doi: 10.1016/j.jtos.2023.06.002. Epub 2023 Jun 23. Ocul Surf. 2023. PMID: 37355021 Free PMC article.
-
The Role of Cystinosin in the Intermediary Thiol Metabolism and Redox Homeostasis in Kidney Proximal Tubular Cells.Antioxidants (Basel). 2018 Dec 3;7(12):179. doi: 10.3390/antiox7120179. Antioxidants (Basel). 2018. PMID: 30513914 Free PMC article. Review.
-
The renal Fanconi syndrome in cystinosis: pathogenic insights and therapeutic perspectives.Nat Rev Nephrol. 2017 Feb;13(2):115-131. doi: 10.1038/nrneph.2016.182. Epub 2016 Dec 19. Nat Rev Nephrol. 2017. PMID: 27990015 Free PMC article. Review.
-
Cystinosin is involved in Na+/H+ Exchanger 3 trafficking in the proximal tubular cells: new insights in the renal Fanconi syndrome in cystinosis.bioRxiv [Preprint]. 2025 Feb 12:2025.02.12.637793. doi: 10.1101/2025.02.12.637793. bioRxiv. 2025. PMID: 39990449 Free PMC article. Preprint.
References
-
- Seatter MJ, Drummond R, Kanke T, Macfarlane SR, Hollenberg MD, Plevin R. The role of the C-terminal tail in protease-activated receptor-2-mediated Ca2+ signalling, proline-rich tyrosine kinase-2 activation, and mitogen-activated protein kinase activity. Cell Signal. 2004;16: 21–29. - PubMed
-
- Chung JJ, Shikano S, Hanyu Y, Li M. Functional diversity of protein C-termini: more than zipcoding? Trends Cell Biol. 2002;12: 146–150. - PubMed
-
- Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18: 319–324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

