A double blind, randomized, active controlled study to assess the safety, tolerability and immunogenicity of measles, mumps rubella, and varicella vaccine (MMRV) manufactured using an alternative process
- PMID: 27149048
- PMCID: PMC4994724
- DOI: 10.1080/21645515.2016.1165374
A double blind, randomized, active controlled study to assess the safety, tolerability and immunogenicity of measles, mumps rubella, and varicella vaccine (MMRV) manufactured using an alternative process
Abstract
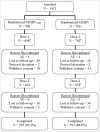
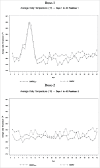
Vaccination against measles, mumps, rubella, and varicella is recommended for all children in the US. Limitations manufacturing Oka/Merck strain varicella-zoster virus have hampered the availability of the combination vaccine (MMRV) against these 4 viruses, which drove the need to investigate an alternative manufacturing process. Healthy children 12-to-23 months of age at 71 US sites were randomized (1:1) to receive MMRV manufactured using an alternative process (MMRVAMP) or the currently licensed MMRV. Subjects received 2 0.5 mL doses 3 months apart. Sera were collected before and 6 weeks after Dose-1. Adverse experiences (AEs) were collected for 42 d after each dose and serious AEs and events of special interest for 180 d after Dose-2. Overall, 706 subjects were randomized to MMRVAMP and 706 to MMRV and 698 and 702 received at least 1 dose of study vaccine, respectively. The risk difference in response rates and geometric mean concentrations of antibody to measles, mumps, rubella, and varicella viruses 6 weeks after Dose-1 met non-inferiority criteria for MMRVAMP versus, MMRV. Response rates met acceptability criteria for each virus, and the seroconversion rate to varicella-zoster virus was 99.5% in both groups. Vaccine-related AEs were mostly mild-to-moderate in intensity and somewhat more common after MMRVAMP. Febrile seizures occurred at similar rates in both groups during the first 42 d after each vaccine dose. MMRVAMP is non-inferior to MMRV and represents an important advancement in maintaining an adequate supply of vaccines against these diseases.
Keywords: MMRV; immunogenicity; measles; mumps; rubella; safety; varicella.
Figures
References
-
- Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States , 2015. http://www.cdc.gov/vaccines/schedules/downloads/child/0–18yrs-schedule.pdf (accessed January25, 2016).
-
- Centers for Disease Control and Prevention (CDC) . Use of combination measles, mumps, rubella, and varicella, vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2010; 59:1-15; PMID:20075837 - PubMed
-
- Centers for Disease Control and Prevention General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2011; 60(RR-2):1-61. - PubMed
-
- U.S. Food an Drug Administration. M-M-R ®II (Measles, Mumps, and Rubella Virus Vaccine Live) [package insert]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedPro...
-
- U.S. Food an Drug Administration. Varivax (Varicella Virus Vaccine Live) [package insert]. Available from : http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedPro...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
