Immunogenicity of Virus Like Particle Forming Baculoviral DNA Vaccine against Pandemic Influenza H1N1
- PMID: 27149064
- PMCID: PMC4858234
- DOI: 10.1371/journal.pone.0154824
Immunogenicity of Virus Like Particle Forming Baculoviral DNA Vaccine against Pandemic Influenza H1N1
Abstract
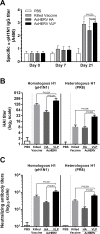
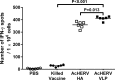
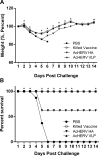
An outbreak of influenza H1N1 in 2009, representing the first influenza pandemic of the 21st century, was transmitted to over a million individuals and claimed 18,449 lives. The current status in many countries is to prepare influenza vaccine using cell-based or egg-based killed vaccine. However, traditional influenza vaccine platforms have several limitations. To overcome these limitations, many researchers have tried various approaches to develop alternative production platforms. One of the alternative approach, we reported the efficacy of influenza HA vaccination using a baculoviral DNA vaccine (AcHERV-HA). However, the immune response elicited by the AcHERV-HA vaccine, which only targets the HA antigen, was lower than that of the commercial killed vaccine. To overcome the limitations of this previous vaccine, we constructed a human endogenous retrovirus (HERV) envelope-coated, baculovirus-based, virus-like-particle (VLP)-forming DNA vaccine (termed AcHERV-VLP) against pandemic influenza A/California/04/2009 (pH1N1). BALB/c mice immunized with AcHERV-VLP (1×10(7) FFU AcHERV-VLP, i.m.) and compared with mice immunized with the killed vaccine or mice immunized with AcHERV-HA. As a result, AcHERV-VLP immunization produced a greater humoral immune response and exhibited neutralizing activity with an intrasubgroup H1 strain (PR8), elicited neutralizing antibody production, a high level of interferon-γ secretion in splenocytes, and diminished virus shedding in the lung after challenge with a lethal dose of influenza virus. In conclusion, VLP-forming baculovirus DNA vaccine could be a potential vaccine candidate capable of efficiently delivering DNA to the vaccinee and VLP forming DNA eliciting stronger immunogenicity than egg-based killed vaccines.
Conflict of interest statement
Figures
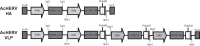

Similar articles
-
Protective efficacy of a human endogenous retrovirus envelope-coated, nonreplicable, baculovirus-based hemagglutin vaccine against pandemic influenza H1N1 2009.PLoS One. 2013 Nov 18;8(11):e80762. doi: 10.1371/journal.pone.0080762. eCollection 2013. PLoS One. 2013. PMID: 24260476 Free PMC article.
-
Effect of AcHERV-GmCSF as an Influenza Virus Vaccine Adjuvant.PLoS One. 2015 Jun 19;10(6):e0129761. doi: 10.1371/journal.pone.0129761. eCollection 2015. PLoS One. 2015. PMID: 26090848 Free PMC article.
-
A novel intranasal virus-like particle (VLP) vaccine designed to protect against the pandemic 1918 influenza A virus (H1N1).Viral Immunol. 2007 Sep;20(3):441-52. doi: 10.1089/vim.2007.0027. Viral Immunol. 2007. PMID: 17931114
-
Antibody Persistence in Adults Two Years after Vaccination with an H1N1 2009 Pandemic Influenza Virus-Like Particle Vaccine.PLoS One. 2016 Feb 26;11(2):e0150146. doi: 10.1371/journal.pone.0150146. eCollection 2016. PLoS One. 2016. PMID: 26919288 Free PMC article. Clinical Trial.
-
Virus-like particle (VLP)-based vaccines for pandemic influenza: performance of a VLP vaccine during the 2009 influenza pandemic.Hum Vaccin Immunother. 2012 Mar;8(3):411-4. doi: 10.4161/hv.18757. Epub 2012 Feb 14. Hum Vaccin Immunother. 2012. PMID: 22330956 Free PMC article. Review.
Cited by
-
Yeast Surface-Displayed H5N1 Avian Influenza Vaccines.J Immunol Res. 2016;2016:4131324. doi: 10.1155/2016/4131324. Epub 2016 Dec 19. J Immunol Res. 2016. PMID: 28078309 Free PMC article.
-
Human endogenous retrovirus-enveloped baculoviral DNA vaccines against MERS-CoV and SARS-CoV2.NPJ Vaccines. 2021 Mar 19;6(1):37. doi: 10.1038/s41541-021-00303-w. NPJ Vaccines. 2021. PMID: 33741992 Free PMC article.
-
Influence of temperature on the antigenic changes of virus-like particles.Clin Exp Vaccine Res. 2020 Jul;9(2):126-132. doi: 10.7774/cevr.2020.9.2.126. Epub 2020 Jul 31. Clin Exp Vaccine Res. 2020. PMID: 32864369 Free PMC article.
-
An Overview of Recent Developments in the Application of Antigen Displaying Vaccine Platforms: Hints for Future SARS-CoV-2 VLP Vaccines.Vaccines (Basel). 2023 Sep 20;11(9):1506. doi: 10.3390/vaccines11091506. Vaccines (Basel). 2023. PMID: 37766182 Free PMC article. Review.
-
Baculovirus capsid display in vaccination schemes: effect of a previous immunity against the vector on the cytotoxic response to delivered antigens.Appl Microbiol Biotechnol. 2018 Dec;102(23):10139-10146. doi: 10.1007/s00253-018-9368-8. Epub 2018 Sep 20. Appl Microbiol Biotechnol. 2018. PMID: 30238142
References
-
- Fabian GT. Pandemic influenza and lessons from history. J S C Med Assoc. 2008;104(5):126–31. Epub 2008/07/30. . - PubMed
-
- Stech J, Beer M, Vahlenkamp T, Harder T. [The pandemic influenza virus H1N1/2009: a review of the molecular biology, phylogeny, history of reassortments, and parameters of host switching]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2010;53(12):1231–7. Epub 2010/12/17. 10.1007/s00103-010-1166-0 . - DOI - PubMed
-
- WHO. World now at the start of 2009 influenza pandemi. WHO—Media centre. 2009. Available: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

