Meta-Analysis on Randomized Controlled Trials of Vaccines with QS-21 or ISCOMATRIX Adjuvant: Safety and Tolerability
- PMID: 27149269
- PMCID: PMC4858302
- DOI: 10.1371/journal.pone.0154757
Meta-Analysis on Randomized Controlled Trials of Vaccines with QS-21 or ISCOMATRIX Adjuvant: Safety and Tolerability
Abstract
Background and objectives: QS-21 shows in vitro hemolytic effect and causes side effects in vivo. New saponin adjuvant formulations with better toxicity profiles are needed. This study aims to evaluate the safety and tolerability of QS-21 and the improved saponin adjuvants (ISCOM, ISCOMATRIX and Matrix-M™) from vaccine trials.
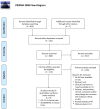
Methods: A systematic literature search was conducted from MEDLINE, EMBASE, Cochrane library and Clinicaltrials.gov. We selected for the meta-analysis randomized controlled trials (RCTs) of vaccines adjuvanted with QS-21, ISCOM, ISCOMATRIX or Matrix-M™, which included a placebo control group and reported safety outcomes. Pooled risk ratios (RRs) and their 95% confidence intervals (CIs) were calculated using a random-effects model. Jadad scale was used to assess the study quality.
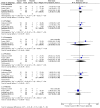
Results: Nine RCTs were eligible for the meta-analysis: six trials on QS-21-adjuvanted vaccines and three trials on ISCOMATRIX-adjuvanted, with 907 patients in total. There were no studies on ISCOM or Matrix-M™ adjuvanted vaccines matching the inclusion criteria. Meta-analysis identified an increased risk for diarrhea in patients receiving QS21-adjuvanted vaccines (RR 2.55, 95% CI 1.04-6.24). No increase in the incidence of the reported systemic AEs was observed for ISCOMATRIX-adjuvanted vaccines. QS-21- and ISCOMATRIX-adjuvanted vaccines caused a significantly higher incidence of injection site pain (RR 4.11, 95% CI 1.10-15.35 and RR 2.55, 95% CI 1.41-4.59, respectively). ISCOMATRIX-adjuvanted vaccines also increased the incidence of injection site swelling (RR 3.43, 95% CI 1.08-10.97).
Conclusions: Our findings suggest that vaccines adjuvanted with either QS-21 or ISCOMATRIX posed no specific safety concern. Furthermore, our results indicate that the use of ISCOMATRIX enables a better systemic tolerability profile when compared to the use of QS-21. However, no better local tolerance was observed for ISCOMATRIX-adjuvanted vaccines in immunized non-healthy subjects. This meta-analysis is limited by the relatively small number of individuals recruited in the included trials, especially in the control groups.
Conflict of interest statement
Figures





Similar articles
-
ISCOMATRIX adjuvant for antigen delivery.Adv Drug Deliv Rev. 2005 Jan 10;57(3):465-74. doi: 10.1016/j.addr.2004.09.006. Adv Drug Deliv Rev. 2005. PMID: 15560952 Review.
-
Saponin-adjuvanted particulate vaccines for clinical use.Methods. 2006 Sep;40(1):53-9. doi: 10.1016/j.ymeth.2006.05.019. Methods. 2006. PMID: 16997713 Review.
-
Immune enhancing properties of the novel Matrix-M™ adjuvant leads to potentiated immune responses to an influenza vaccine in mice.Vaccine. 2013 Mar 25;31(13):1725-33. doi: 10.1016/j.vaccine.2013.01.039. Epub 2013 Feb 4. Vaccine. 2013. PMID: 23384754
-
Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems.Expert Rev Vaccines. 2011 Apr;10(4):471-86. doi: 10.1586/erv.11.29. Expert Rev Vaccines. 2011. PMID: 21506645 Review.
-
ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines.Expert Rev Vaccines. 2007 Oct;6(5):761-72. doi: 10.1586/14760584.6.5.761. Expert Rev Vaccines. 2007. PMID: 17931156 Review.
Cited by
-
Performance of a Four-Antigen Staphylococcus aureus Vaccine in Preclinical Models of Invasive S. aureus Disease.Microorganisms. 2021 Jan 15;9(1):177. doi: 10.3390/microorganisms9010177. Microorganisms. 2021. PMID: 33467609 Free PMC article.
-
Leaf Saponins of Quillaja brasiliensis as Powerful Vaccine Adjuvants.Pharmaceutics. 2025 Jul 25;17(8):966. doi: 10.3390/pharmaceutics17080966. Pharmaceutics. 2025. PMID: 40870989 Free PMC article. Review.
-
Novel adjuvants in allergen-specific immunotherapy: where do we stand?Front Immunol. 2024 Feb 23;15:1348305. doi: 10.3389/fimmu.2024.1348305. eCollection 2024. Front Immunol. 2024. PMID: 38464539 Free PMC article. Review.
-
Quillaja brasiliensis nanoparticle adjuvant formulation improves the efficacy of an inactivated trivalent influenza vaccine in mice.Front Immunol. 2023 May 1;14:1163858. doi: 10.3389/fimmu.2023.1163858. eCollection 2023. Front Immunol. 2023. PMID: 37197659 Free PMC article.
-
Potentials of saponins-based adjuvants for nasal vaccines.Front Immunol. 2023 Mar 20;14:1153042. doi: 10.3389/fimmu.2023.1153042. eCollection 2023. Front Immunol. 2023. PMID: 37020548 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

