Cardiothoracic Applications of 3-dimensional Printing
- PMID: 27149367
- PMCID: PMC4993676
- DOI: 10.1097/RTI.0000000000000217
Cardiothoracic Applications of 3-dimensional Printing
Abstract
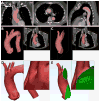
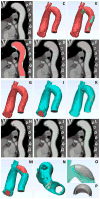
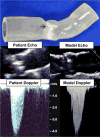
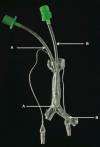
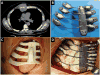
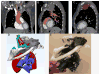
Medical 3-dimensional (3D) printing is emerging as a clinically relevant imaging tool in directing preoperative and intraoperative planning in many surgical specialties and will therefore likely lead to interdisciplinary collaboration between engineers, radiologists, and surgeons. Data from standard imaging modalities such as computed tomography, magnetic resonance imaging, echocardiography, and rotational angiography can be used to fabricate life-sized models of human anatomy and pathology, as well as patient-specific implants and surgical guides. Cardiovascular 3D-printed models can improve diagnosis and allow for advanced preoperative planning. The majority of applications reported involve congenital heart diseases and valvular and great vessels pathologies. Printed models are suitable for planning both surgical and minimally invasive procedures. Added value has been reported toward improving outcomes, minimizing perioperative risk, and developing new procedures such as transcatheter mitral valve replacements. Similarly, thoracic surgeons are using 3D printing to assess invasion of vital structures by tumors and to assist in diagnosis and treatment of upper and lower airway diseases. Anatomic models enable surgeons to assimilate information more quickly than image review, choose the optimal surgical approach, and achieve surgery in a shorter time. Patient-specific 3D-printed implants are beginning to appear and may have significant impact on cosmetic and life-saving procedures in the future. In summary, cardiothoracic 3D printing is rapidly evolving and may be a potential game-changer for surgeons. The imager who is equipped with the tools to apply this new imaging science to cardiothoracic care is thus ideally positioned to innovate in this new emerging imaging modality.
Conflict of interest statement
Dr. Mitsouras receives research support from Vital Images, A Toshiba Medical Systems Group Company
Figures










References
-
- Markl M, Schumacher R, Kuffer J, et al. Rapid vessel prototyping: vascular modeling using 3t magnetic resonance angiography and rapid prototyping technology. MAGMA. 2005;18(6):288–92. - PubMed
-
- Noecker AM, Chen JF, Zhou Q, et al. Development of patient-specific three-dimensional pediatric cardiac models. ASAIO J. 2006;52(3):349–53. - PubMed
-
- Schievano S, Sala G, Migliavacca F, et al. Use of rapid prototyping models in the planning of percutaneous pulmonary valved stent implantation. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2007;221(4):407–416. - PubMed
-
- Mottl-Link S, Hubler M, Kuhne T, et al. Physical models aiding in complex congenital heart surgery. Ann Thorac Surg. 2008;86(1):273–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

