Corticosteroids for the treatment of Duchenne muscular dystrophy
- PMID: 27149418
- PMCID: PMC8580515
- DOI: 10.1002/14651858.CD003725.pub4
Corticosteroids for the treatment of Duchenne muscular dystrophy
Abstract
Background: Duchenne muscular dystrophy (DMD) is the most common muscular dystrophy of childhood. Untreated, this incurable disease, which has an X-linked recessive inheritance, is characterised by muscle wasting and loss of walking ability, leading to complete wheelchair dependence by 13 years of age. Prolongation of walking is a major aim of treatment. Evidence from randomised controlled trials (RCTs) indicates that corticosteroids significantly improve muscle strength and function in boys with DMD in the short term (six months), and strength at two years (two-year data on function are very limited). Corticosteroids, now part of care recommendations for DMD, are largely in routine use, although questions remain over their ability to prolong walking, when to start treatment, longer-term balance of benefits versus harms, and choice of corticosteroid or regimen.We have extended the scope of this updated review to include comparisons of different corticosteroids and dosing regimens.
Objectives: To assess the effects of corticosteroids on prolongation of walking ability, muscle strength, functional ability, and quality of life in DMD; to address the question of whether benefit is maintained over the longer term (more than two years); to assess adverse events; and to compare efficacy and adverse effects of different corticosteroid preparations and regimens.
Search methods: On 16 February 2016 we searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, EMBASE, CINAHL Plus, and LILACS. We wrote to authors of published studies and other experts. We checked references in identified trials, handsearched journal abstracts, and searched trials registries.
Selection criteria: We considered RCTs or quasi-RCTs of corticosteroids (e.g. prednisone, prednisolone, and deflazacort) given for a minimum of three months to patients with a definite DMD diagnosis. We considered comparisons of different corticosteroids, regimens, and corticosteroids versus placebo.
Data collection and analysis: The review authors followed standard Cochrane methodology.
Main results: We identified 12 studies (667 participants) and two new ongoing studies for inclusion. Six RCTs were newly included at this update and important non-randomised cohort studies have also been published. Some important studies remain unpublished and not all published studies provide complete outcome data.
Primary outcome measure: one two-year deflazacort RCT (n = 28) used prolongation of ambulation as an outcome measure but data were not adequate for drawing conclusions.
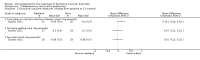
Secondary outcome measures: meta-analyses showed that corticosteroids (0.75 mg/kg/day prednisone or prednisolone) improved muscle strength and function versus placebo over six months (moderate quality evidence from up to four RCTs). Evidence from single trials showed 0.75 mg/kg/day superior to 0.3 mg/kg/day on most strength and function measures, with little evidence of further benefit at 1.5 mg/kg/day. Improvements were seen in time taken to rise from the floor (Gowers' time), timed walk, four-stair climbing time, ability to lift weights, leg function grade, and forced vital capacity. One new RCT (n = 66), reported better strength, function and quality of life with daily 0.75 mg/kg/day prednisone at 12 months. One RCT (n = 28) showed that deflazacort stabilised muscle strength versus placebo at two years, but timed function test results were too imprecise for conclusions to be drawn.One double-blind RCT (n = 64), largely at low risk of bias, compared daily prednisone (0.75 mg/kg/day) with weekend-only prednisone (5 mg/kg/weekend day), finding no overall difference in muscle strength and function over 12 months (moderate to low quality evidence). Two small RCTs (n = 52) compared daily prednisone 0.75 mg/kg/day with daily deflazacort 0.9 mg/kg/day, but study methods limited our ability to compare muscle strength or function.
Adverse effects: excessive weight gain, behavioural abnormalities, cushingoid appearance, and excessive hair growth were all previously shown to be more common with corticosteroids than placebo; we assessed the quality of evidence (for behavioural changes and weight gain) as moderate. Hair growth and cushingoid features were more frequent at 0.75 mg/kg/day than 0.3 mg/kg/day prednisone. Comparing daily versus weekend-only prednisone, both groups gained weight with no clear difference in body mass index (BMI) or in behavioural changes (low quality evidence for both outcomes, one study); the weekend-only group had a greater linear increase in height. Very low quality evidence suggested less weight gain with deflazacort than with prednisone at 12 months, and no difference in behavioural abnormalities. Data are insufficient to assess the risk of fractures or cataracts for any comparison.Non-randomised studies support RCT evidence in showing improved functional benefit from corticosteroids. These studies suggest sustained benefit for up to 66 months. Adverse effects were common, although generally manageable. According to a large comparative longitudinal study of daily or intermittent (10 days on, 10 days off) corticosteroid for a mean period of four years, a daily regimen prolongs ambulation and improves functional scores over the age of seven, but with a greater frequency of side effects than an intermittent regimen.
Authors' conclusions: Moderate quality evidence from RCTs indicates that corticosteroid therapy in DMD improves muscle strength and function in the short term (twelve months), and strength up to two years. On the basis of the evidence available for strength and function outcomes, our confidence in the effect estimate for the efficacy of a 0.75 mg/kg/day dose of prednisone or above is fairly secure. There is no evidence other than from non-randomised trials to establish the effect of corticosteroids on prolongation of walking. In the short term, adverse effects were significantly more common with corticosteroids than placebo, but not clinically severe. A weekend-only prednisone regimen is as effective as daily prednisone in the short term (12 months), according to low to moderate quality evidence from a single trial, with no clear difference in BMI (low quality evidence). Very low quality evidence indicates that deflazacort causes less weight gain than prednisone after a year's treatment. We cannot evaluate long-term benefits and hazards of corticosteroid treatment or intermittent regimens from published RCTs. Non-randomised studies support the conclusions of functional benefits, but also identify clinically significant adverse effects of long-term treatment, and a possible divergence of efficacy in daily and weekend-only regimens in the longer term. These benefits and adverse effects have implications for future research and clinical practice.
Conflict of interest statement
Dr Emma Matthews has no conflicts of interest.
Dr Ruth Brassington is Managing Editor of Cochrane Neuromuscular, of which The National Institute for Health Research (NIHR) is the largest single funder. The NIHR provided an incentive award to Cochrane Neuromuscular for the updating of this review (see Acknowledgements). A grant from the Motor Neurone Disease Association to Cochrane Neuromuscular contributed to her salary in 2011‐2015. She has no financial conflicts of interest. She withdrew from the later stages of the editorial process of this review.
Dr Thierry Kuntzer has no conflicts of interest.
Fatima Jichi has no known conflicts of interest.
Dr Adnan Y Manzur, at the time of preparation and submission of the protocol for this review was the principal investigator of a proposed UK multicentre trial of prednisolone in Duchenne muscular dystrophy. However, this trial was not funded. Currently, Dr Manzur is the lead clinician of the UK North Star Clinical Network for Neuromuscular Disorders. The clinicians on this clinical network have a consensus on approach to use of corticosteroids (prednisolone) and plans for future collaboration to audit and modify clinical practice in line with available evidence.
Figures






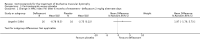

























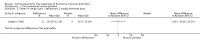
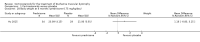
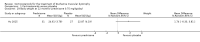
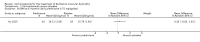






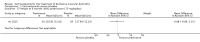


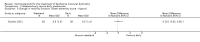


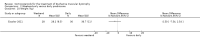
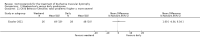
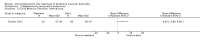
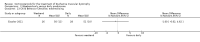




Update of
-
Glucocorticoid corticosteroids for Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2008 Jan 23;(1):CD003725. doi: 10.1002/14651858.CD003725.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2016 May 05;(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. PMID: 18254031 Updated.
References
References to studies included in this review
Angelini 1994 {published data only}
-
- Angelini C, Pegoraro E, Turella E, Intino MT, Pini A, Costa C. Deflazacort in Duchenne dystrophy: study of long‐term effect. Muscle & Nerve 1994;17(4):386‐91. [PUBMED: 8170484] - PubMed
Bäckman 1995 {published data only}
-
- Bäckman E, Henriksson KG. Low‐dose prednisolone treatment in Duchenne and Becker muscular dystrophy. Neuromuscular Disorders 1995;5(3):233‐41. [PUBMED: 7633189] - PubMed
Beenakker 2005 {published data only}
-
- Beenakker EA, Fock JM, Tol MJ, Maurits NM, Koopman HM, Brouwer OF, et al. Intermittent prednisone therapy in Duchenne muscular dystrophy: a randomized controlled trial. Archives of Neurology 2005;62(1):128‐32. [PUBMED: 15642859] - PubMed
Bonifati 2000 {published data only}
-
- Bonifati MD, Ruzza G, Bonometto P, Berardinelli A, Gorni K, Orcesi S, et al. A multicenter, double‐blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle & Nerve 2000;23(9):1344‐7. - PubMed
Brooke 1996 {published data only}
-
- Brooke MH. A randomised trial of deflazacort and prednisone in Duchenne muscular dystrophy: efficacy and toxicity. Neurology 1996;46:A476.
Escolar 2011 {published and unpublished data}
-
- Escolar D, McDonald C, Kornberg AJ, Bertorini T, Nevo Y, Lotze T. Randomized, double‐blind, controlled study to compare efficacy and tolerability of standard daily prednisone regime with a novel intermittent high dose regime in ambulant boys with Duchenne muscular dystrophy. Neurology 2008; Vol. 70, issue 11 Suppl 1:A109‐10, Abstract no: S05.004.
Griggs 1991 {published data only}
-
- Griggs RC, Moxley RT 3rd, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Prednisone in Duchenne dystrophy. A randomized controlled trial defining the course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Archives of Neurology 1991;48(4):383‐8. [PUBMED: 2012511] - PubMed
Hu 2015 {published data only}
-
- Hu J, Ye Y, Kong M, Hong S, Cheng L, Wang Q, et al. Daily prednisone treatment in Duchenne muscular dystrophy in southwest China. Muscle & Nerve 2015;52(6):1001–7. [PUBMED: 25809413] - PubMed
Karimzadeh 2012 {published data only}
-
- Karimzadeh P, Ghazavi A. Comparison of deflazacort and prednisone in Duchenne muscular dystrophy. Iranian Journal of Child Neurology 2012; Vol. 6, issue 1:5‐12.
Mendell 1989 {published data only}
-
- Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, et al. Randomized, double‐blind six‐month trial of prednisone in Duchenne's muscular dystrophy. New England Journal of Medicine 1989;320(24):1592‐7. [PUBMED: 2657428] - PubMed
Rahman 2001 {published and unpublished data}
-
- Rahman MM, Hannan MM, Mondol BA, Bhoumick NB, Haque A. Prednisolone in Duchenne muscular dystrophy. Bangladesh Medical Research Council Bulletin 2001;27(1):38‐42. [PUBMED: 11692899] - PubMed
Todorovic 1998 {published data only}
-
- Todorovic SM. High dose (2 mg/kg) alternate day prednisone therapy in the treatment of Duchenne muscular dystrophy. Muscle & Nerve. 1998; Vol. 7 Suppl:72.
References to studies excluded from this review
Ahlander 2003 {published data only}
-
- Ahlander AC, Kroksmark AK, Tulinius M. Low‐dosage prednisolone in the long‐term treatment of Duchenne muscular dystrophy. Neuromuscular Disorders 2003;13(7‐8):630.
Alman 2004 {published data only}
-
- Alman BA, Raza NS, Biggar WD. Steroid treatment and the development of scoliosis in males with Duchenne muscular dystrophy. Journal of Bone and Joint Surgery. American Volume 2004;86‐A(3):519‐24. - PubMed
Angelini 1995 {published data only}
-
- Angelini C, Pegoraro E, Cadaldini M. Daily versus alternate‐day deflazacort (DFZ) in Duchenne muscular dystrophy. Neurology 1995;45(Suppl 4):A182.
Angelini 2007 {published data only}
-
- Angelini C. The role of corticosteroids in muscular dystrophy: a critical appraisal. Muscle & Nerve 2007;36(4):424‐35. - PubMed
Angelini 2012 {published data only}
Aviles 1982 {published data only}
-
- Luz Aviles C, Gutiérrez C, Novoa F, Gil E, Stuardo A. Steroid treatment of Duchenne's muscular dystrophy [Tratamiento esteroidal en distrofia muscular de Duchenne]. Revista Chilena de Pediatra 1982;53(3):187‐91. - PubMed
Balaban 2005 {published data only}
-
- Balaban B, Matthews DJ, Clayton GH, Carry T. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long‐term effect. American Journal of Physical Medicine and Rehabilitation 2005;84(11):843‐50. - PubMed
Biggar 2001 {published data only}
-
- Biggar WD, Gingras M, Fehlings DL, Harris VA, Steele CA. Deflazacort treatment of Duchenne muscular dystrophy. Journal of Pediatrics 2001;138(1):45‐50. - PubMed
Biggar 2004 {published data only}
-
- Biggar WD, Politano L, Harris VA, Passamano L, Vajsar J, Alman B, et al. Deflazacort in Duchenne muscular dystrophy: a comparison of two different protocols. Neuromuscular Disorders 2004;14(8‐9):476‐82. - PubMed
Biggar 2006 {published data only}
-
- Biggar WD, Harris VA, Eliasoph L, Alman B. Long‐term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscular Disorders 2006;16(4):249‐55. - PubMed
Bonifati 2006 {published data only}
Bothwell 2003 {published data only}
-
- Bothwell JE, Gordon KE, Dooley JM, MacSween J, Cummings EA, Salisbury S. Vertebral fractures in boys with Duchenne muscular dystrophy. Clinical Pediatrics 2003;42(4):353‐6. - PubMed
Brooke 1987 {published data only}
-
- Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley RT 3rd, Miller JP, et al. Clinical investigation of Duchenne muscular dystrophy: interesting results in a trial of prednisone. Archives of Neurology 1987;44(8):812‐7. - PubMed
Campbell 2003 {published data only}
Connolly 2002 {published data only}
-
- Connolly AM, Schierbecker J, Renna R, Florence J. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. Neuromuscular Disorders 2002;12(10):917‐25. - PubMed
Daftary 2007 {published data only}
-
- Daftary AS, Crisanti M, Kalra M, Wong B, Amin R. Effect of long‐term steroids on cough efficiency and respiratory muscle strength in patients with Duchenne muscular dystrophy. Pediatrics 2007;119(2):e320‐4. - PubMed
de Groot 2002 {published data only}
-
- Groot IJM. The effectiveness of prednisolone treatment (10 days on/10 days off) in the ambulatory phase of Duchenne muscular dystrophy: an open study. Neuromuscular Disorders 2002;12(7‐8):737‐8.
DeSilva 1987 {published data only}
-
- DeSilva S, Drachman DB, Mellits D, Kuncl R. Prednisone treatment in Duchenne muscular dystrophy. Long‐term benefit. Archives of Neurology 1987;44(8):818‐22. - PubMed
Drachman 1974 {published data only}
-
- Drachman DB, Toyka KV, Myer E. Prednisone in Duchenne muscular dystrophy. Lancet 1974;2(7894):1409‐12. - PubMed
Dubowitz 2002 {published data only}
-
- Dubowitz V, Kinali M, Main M, Mercuri E, Muntoni F. Remission of clinical signs in early Duchenne muscular dystrophy on intermittent low‐dosage prednisolone therapy. European Journal of Paediatric Neurology 2002;6(3):153‐9. - PubMed
Dubrovsky 1999 {published data only}
-
- Dubrovsky AL, Vito E, Suarez A, Mesa LE, Pessolano F, Sobrino R, et al. Deflazacort treatment and respiratory function in Duchenne muscular dystrophy. Neurology 1999;52 Suppl(2):A544.
Fenichel 1991a {published data only}
-
- Fenichel GM, Mendell JR, Moxley RT, Griggs RC, Brooke MH, Miller JP. A comparison of daily and alternate‐day prednisone therapy in the treatment of Duchenne muscular dystrophy. Archives of Neurology 1991;48(6):575‐9. - PubMed
Fenichel 1991b {published data only}
-
- Fenichel GM, Florence JM, Pestronk A, Mendell JR, Moxley RT 3rd, Griggs RC, et al. Long‐term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology 1991;41(12):1874‐7. - PubMed
Flanigan 2012 {published data only}
-
- Flanigan KM. The muscular dystrophies. Seminars in Neurology 2012;32(3):255‐63. - PubMed
Griggs 1993 {published data only}
-
- Griggs RC, Moxley RT 3rd, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Duchenne dystrophy: randomized, controlled trial of prednisone (18 months) and azathioprine (12 months). Neurology 1993;43(3 Pt 1):520‐7. - PubMed
Griggs 2013 {published data only}
-
- Griggs RC, Herr BE, Reha A, Elfring G, Atkinson L, Cwik V, et al. Corticosteroids in Duchenne muscular dystrophy: major variations in practice. Muscle & Nerve 2013; Vol. 48, issue 1:27‐31. - PubMed
Henricson 2013 {published data only}
-
- Henricson EK, Abresch RT, Cnaan A, Hu F, Duong T, Arrieta A, et al. CINRG Investigators. The Cooperative International Neuromuscular Research Group Duchenne Natural History Study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle & Nerve 2013;48(1):55–67. - PMC - PubMed
Houde 2008 {published data only}
-
- Houde S, Filiatrault M, Fournier A, Dubé J, D'Arcy S, Bérubé D, et al. Deflazacort use in Duchenne muscular dystrophy: an 8‐year follow‐up. Pediatric Neurology 2008;38(3):200‐6. - PubMed
Kinali 2002 {published data only}
-
- Kinali M, Mercuri E, Main M, Muntoni F, Dubowitz V. An effective, low‐dosage, intermittent schedule of prednisolone in the long‐term treatment of early cases of Duchenne dystrophy. Neuromuscular Disorders 2002;12(Suppl 1):S169‐74. - PubMed
Kinali 2007 {published data only}
-
- Kinali M, Main M, Eliahoo J, Messina S, Knight RK, Lehovsky J. Predictive factors for the development of scoliosis in Duchenne muscular dystrophy. European Journal of Paediatric Neurology 2007;11(3):160‐6. - PubMed
King 2007 {published data only}
-
- King WM, Ruttencutter R, Nagaraja HN, Matkovic V, Landoll J, Hoyle C. Orthopedic outcomes of long‐term daily dose corticosteroid treatment in Duchenne muscular dystrophy. Neurology 2007;68(19):1607‐13. - PubMed
Markham 2005 {published data only}
-
- Markham LW, Spicer RL, Khoury PR, Wong BL, Mathews KD, Cripe LH. Steroid therapy and cardiac function in Duchenne muscular dystrophy. Pediatric Cardiology 2005;26(6):768‐71. [10.1007/s00246‐005‐0909‐4] - PubMed
Mayhew 2013 {published data only}
-
- Mayhew AG, Cano SJ, Scott E, Eagle M, Bushby K, Manzur A, et al. Detecting meaningful change using the North Star Ambulatory Assessment in Duchenne muscular dystrophy. Developmental Medicine and Child Neurology 2013; Vol. 55, issue 11:1046‐52. - PubMed
Mazzone 2013 {published data only}
McAdam 2012 {published data only}
Merlini 2003 {published data only}
-
- Merlini L, Cicognani A, Malaspina E, Gennari M, Gnudi S, Talim B, et al. Early prednisone treatment in Duchenne muscular dystrophy. Muscle & Nerve 2003;27(2):222‐7. - PubMed
Mesa 1991 {published data only}
-
- Mesa LE, Dubrovsky AL, Corderi J, Marco P, Flores D. Steroids in Duchenne muscular dystrophy ‐ deflazacort trial. Neuromuscular Disorders 1991;1(4):261‐6. - PubMed
Pandya 2001 {published data only}
-
- Pandya S, Myers G, Moxley RT. Effect of daily prednisone on independent ambulation in patients with Duchenne dystrophy treated for up to 15 years. Neuromuscular Disorders 2001;11(6‐7):630.
Parreira 2007 {published data only}
-
- Parreira SL, Resende MB, Della Corte Peduto M, Marie SK, Carvalho MS, Reed UC. Quantification of muscle strength and motor ability in patients with Duchenne muscular dystrophy on steroid therapy. Arquivos de Neuro‐Psiquiatria 2007;65(2A):245‐50. - PubMed
Pradhan 2006 {published data only}
-
- Pradhan S, Ghosh D, Srivastava NK, Kumar A, Mittal B, Pandey CM, et al. Prednisolone in Duchenne muscular dystrophy with imminent loss of ambulation. Journal of Neurology 2006;253(10):1309‐16. [DOI 10.1007/s00415‐006‐0212‐1] - PubMed
Reitter 1995 {published and unpublished data}
-
- Reitter B. Deflazacort vs. prednisone in Duchenne muscular dystrophy: trends of an ongoing study. Brain & Development 1995;17 Suppl:39‐43. - PubMed
Resende 2001 {published data only}
-
- Resende MBD, Reed UC, Espindola AA, Ferreira LG, Carvalho MS, Diament A, et al. Deflazacort in Duchenne muscular dystrophy: preliminary results in a Brazilian series. Neuromuscular Disorders 2001;11:630.
Ricotti 2013 {published data only}
-
- Ricotti V, Ridout DA, Scott E, Quinlivan R, Robb SA, Manzur AY, et al. NorthStar Clinical Network. Long‐term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. Journal of Neurology, Neurosurgery & Psychiatry 2013;84(6):698‐705. - PubMed
Sansome 1993 {published data only}
-
- Sansome A, Royston P, Dubowitz V. Steroids in Duchenne muscular dystrophy; pilot study of a new low‐dosage schedule. Neuromuscular Disorders 1993;3(5‐6):567‐9. - PubMed
Schara 2001 {published data only}
-
- Schara U, Mortier J, Mortier W. Long‐term steroid therapy in Duchenne muscular dystrophy‐positive results versus side effects. Journal of Clinical Neuromuscular Disease 2001;2(4):179‐83. - PubMed
Schram 2013 {published data only}
-
- Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M, et al. All‐cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. Journal of the American College of Cardiology 2013; Vol. 61, issue 9:948‐54. - PubMed
Siegel 1974 {published data only}
-
- Siegel IM, Miller JE, Ray RD. Failure of corticosteroid in the treatment of Duchenne (pseudo‐hypertrophic) muscular dystrophy. Report of a clinically matched three year double‐blind study. Illinois Medical Journal 1974;145(1):32‐3. - PubMed
Silva 2012 {published data only}
-
- Silva EC, Machado DL, Resende MB, Silva RF, Zanoteli E, Reed UC. Motor function measure scale, steroid therapy and patients with Duchenne muscular dystrophy. Arquivos de Neuro‐psiquiatria 2012; Vol. 70, issue 3:191‐5. - PubMed
Silversides 2003 {published data only}
-
- Silversides CK, Webb GD, Harris VA, Biggar DW. Effects of deflazacort on left ventricular function in patients with Duchenne muscular dystrophy. American Journal of Cardiology 2003;91(6):769‐72. - PubMed
Simon 2011 {published data only}
-
- Simon VA, Resende MB, Simon MA, Zanoteli E, Reed UC. Duchenne muscular dystrophy: quality of life among 95 patients evaluated using the Life Satisfaction Index for Adolescents. Arquivos de Neuro‐psiquiatria 2011; Vol. 69, issue 1:19‐22. - PubMed
Takeuchi 2013 {published data only}
Tunca 2001 {published data only}
-
- Tunca O, Kabakus O, Herguner A, Karaduman A, Topaloglu H. Alternate day prednisone therapy in Duchenne muscular dystrophy. Neuromuscular Disorders 2001;11:630.
Vasanth 1996 {published data only}
-
- Vasanth A, Gourie‐Devi M, Rajaram P, Taly AB, Venkataram BS, Ravishankar D, et al. Duchenne muscular dystrophy: therapeutic options and rehabilitation. European Journal of Neurology 1996;3(Suppl 2):20.
Wong 2002 {published data only}
-
- Wong BL, Christopher C. Corticosteroids in Duchenne muscular dystrophy: a reappraisal. Journal of Child Neurology 2002;17(3):183‐9. - PubMed
Yilmaz 2004 {published data only}
-
- Yilmaz O, Karaduman A, Aras O, Basoglu B, Topaloğlu H. Prednisolone therapy in Duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. Neuromuscular Disorders 2004; Vol. 14:581. - PubMed
-
- Yilmaz O, Karaduman A, Topaloğlu H. Prednisolone therapy in Duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. European Journal of Neurology 2004;11(8):541‐4. - PubMed
References to studies awaiting assessment
ACTRN12605000075684 {published data only}
-
- ACTRN12605000075684. A randomized phase III study to evaluate the effectiveness of two different dosing regimens (high dose vs daily) of prednisone for boys with Duchenne muscular dystrophy in improving muscle strength and function and minimising side effects. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12605000075684 (accessed 26 April 2016).
Bello 2015 {published data only}
-
- Bello L, Kesari A, Gordish‐Dressman H, Cnaan A, Morgenroth LP, Punetha J, et al. Cooperative International Neuromuscular Research Group Investigators. Genetic modifiers of ambulation in the Cooperative International Neuromuscular Research Group Duchenne Natural History Study. Annals of Neurology 2015;77(4):684‐96. - PMC - PubMed
References to ongoing studies
CTRI/2009/091/000738 {published data only}
-
- CTRI/2009/091/000738. A clinical trial to compare the two ways of giving steroids (daily versus intermittent) in ambulatory patients with Duchenne muscular dystrophy. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2009/091/000605 accessed 28 April 2016.
Guglieri 2015 {published data only}
-
- Guglieri M, Ruiten HJA, Speed C, Hart K, Watson G, McColl E, et al. FOR‐DMD: Double‐blind randomised trial to optimise corticosteroid regime in Duchenne muscular dystrophy (DMD). Developmental Medicine and Child Neurology 2015; Vol. 57, issue Suppl s1:25‐6.
-
- NCT01603407. Finding the optimum regimen for Duchenne muscular dystrophy (FOR‐DMD). www.clinicaltrials.gov/ct2/show/NCT01603407 (accessed 14 December 2015).
Additional references
Allsop 1981
-
- Allsop KG, Ziter FA. Loss of strength and functional decline in Duchenne dystrophy. Archives of Neurology 1981;38(7):406‐11. - PubMed
Anderson 2000
-
- Anderson JE, Weber M, Vargus C. Deflazacort increases laminin expression and myogenic repair, and induces early persistent functional gain in mdx mouse muscular dystrophy. Cell Transplantation 2000;9(4):551‐64. - PubMed
Arahata 1984
-
- Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies. I: Quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Annals of Neurology 1984;16(2):193‐208. - PubMed
Azarnoff 1975
-
- Azarnoff DL. Steroid Therapy. 1st Edition. Philadelphia: Saunders, 1975.
Bal 1980
-
- Bal E, Sanwall W. A synergistic effect of glucocorticosteroids and insulin on the differentiation of myoblasts. Journal of Cell Physiology 1980;102:27‐36. - PubMed
Beenakker 2001
-
- Beenakker EAC, Hoeven JH, Fock JM, Maurits NM. Reference values of maximum isometric muscle force obtained in 270 children aged 4 ‐16 years by hand‐held dynamometry. Neuromuscular Disorders 2001;11(5):441‐6. - PubMed
Beenakker 2005b
-
- Beenakker EAC, Maurits NM, Fock JM, Brouwer OF, Hoeven JH. Functional ability and muscle force in healthy children and ambulant Duchenne muscular dystrophy patients. European Journal of Paediatric Neurology 2005;9(6):387–93. - PubMed
Biggar 2005
-
- Biggar WD, Bachrach LK, Henderson RC, Kalkwarf H, Plotkin H, Wong BL. Bone health in Duchenne muscular dystrophy: a workshop report from the meeting in Cincinnati, Ohio, July 8, 2004. Neuromuscular Disorders 2005;15(1):80‐5. - PubMed
BNF 2016
-
- Joint Formulary Committee. British National Formulary (online). www.medicinescomplete.com (accessed 4 April 2016).
Brooke 1981
-
- Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle & Nerve 1981;4(3):186‐97. - PubMed
Brooke 1983
-
- Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Province MA. Clinical investigation in Duchenne dystrophy. 2. Determination of the 'power' of therapeutic trials based on natural history. Muscle & Nerve 1983;6(2):91‐103. - PubMed
Bushby 2003
-
- Bushby K, Muntoni F, Bourke JP. 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th‐9th June 2002, Naarden, the Netherlands. Neuromuscular Disorders 2003;13(2):166‐72. - PubMed
Bushby 2004
-
- Bushby K, Muntoni F, Urtizberea, Hughes R, Griggs R. Report on the 124th ENMC International Workshop. Treatment of Duchenne muscular dystrophy; defining the gold standards of management in the use of corticosteroids. 2‐4 April 2004, Naarden, The Netherlands. Neuromuscular Disorders 2004;14(8‐9):526‐34. - PubMed
Bushby 2007
-
- K Bushby, R Griggs, MSG/ENMC for DMD Trial Study Group. 145th ENMC International Workshop: Planning for an International Trial of Steroid Dosage Regimes in DMD (FOR DMD). 22‐24th October 2006, Naarden, The Netherlands. Neuromuscular Disorders 2007;17(5):423‐8. - PubMed
Bushby 2010a
-
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurology 2010;9(1):77‐93. - PubMed
Bushby 2010b
-
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurology 2010;9(2):177‐89. - PubMed
Davis 2010
-
- Davis SE, Hynan LS, Limbers CA, Andersen CM, Greene MC, Varni JW, et al. The PedsQL in pediatric patients with Duchenne muscular dystrophy: feasibility, reliability, and validity of the Pediatric Quality of Life Inventory Neuromuscular Module and Generic Core Scales. Journal of Clinical Neuromuscular Disease 2010;11(3):97‐109. - PubMed
Deconinck 2007
-
- Deconinck N, Dan B. Pathophysiology of Duchenne muscular dystrophy: Current hypotheses. Pediatric Neurology 2007;36(1):1‐7. - PubMed
Dubowitz 1978
-
- Dubowitz V. Muscle Disorders in Childhood. 2nd Edition. Philadelphia: Saunders, 1978.
Dubowitz 1991
-
- Dubowitz V. Prednisone in Duchenne dystrophy. Neuromuscular Disorders 1991;1(3):161‐3. - PubMed
Dubowitz 1995
-
- Dubowitz V. Muscle Disorders in Childhood. 2nd Edition. London: Saunders, 1995.
Dubowitz 2000
-
- Dubowitz V. 75th European Neuromuscular Centre International workshop: 2nd workshop on the treatment of muscular dystrophy, 10‐12 December, 1999, Naarden, The Netherlands. Neuromuscular Disorders 2000;10(4‐5):313‐20. - PubMed
Dubowitz 2005
-
- Dubowitz V. Prednisone for Duchenne muscular dystrophy. The Lancet Neurology 2005;4(5):264. - PubMed
Dubrovsky 1998
-
- Dubrovsky AL, Angelini C, Bonifati DM, Pegoraro E, Mesa L. Steroids in muscular dystrophy: Where do we stand?. Neuromuscular Disorders 1998;8(6):380‐4. - PubMed
Dudley 2006
Eagle 2002
-
- Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscular Disorders 2002;12(10):926‐9. - PubMed
Eagle 2007
-
- Eagle M, Bourke J, Bullock R, Gibson M, Mehta J, Giddings D, et al. Managing Duchenne muscular dystrophy ‐ the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscular Disorders 2007;17(6):470‐5. - PubMed
Elia 1981
Emery 1991
-
- Emery AEH. Population frequencies of inherited neuromuscular diseases: a world survey. Neuromuscular Disorders 1991;1(1):19‐29. - PubMed
Emery 1997
-
- Emery AEH. Diagnostic Criteria for Neuromuscular Disorders. 2nd Edition. London: Royal Society of Medicine Press, 1997.
Emery 2003
-
- Emery AEH, Muntoni F. Duchenne muscular dystrophy. Oxford Monographs on Medical Genetics. 3rd Edition. Oxford: Oxford Medical Publications, 2003.
Engel 1982
-
- Engel AG, Biesecker G. Complement activation in muscle fiber necrosis: demonstration of the membrane attack complex of complement in necrotic fibers. Annals of Neurology 1982;12(3):289‐96. - PubMed
Frankel 1976
-
- Frankel K, Rosser R. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Human Pathology 1976;7(4):375‐86. - PubMed
Frey 1990
-
- Frey BM, Frey FJ. Clinical pharmacokinetics of prednisone and prednisolone. Clinical Pharmacokinetics 1990;19(2):126‐46. - PubMed
Gardner‐Medwin 1980
-
- Gardner‐Medwin D. Clinical features and classification of the muscular dystrophies. British Medical Bulletin 1980;36(2):109‐15. - PubMed
Gloss 2016
Gomez‐Merino 2002
-
- Gomez‐Merino E, Bach JR. Duchenne muscular dystrophy: prolongation of life by noninvasive ventilation and mechanically assisted coughing. American Journal of Physical and Medical Rehabilitation 2002;81(6):411‐5. - PubMed
Heckmatt 1985
-
- Heckmatt JZ, Dubowitz V, Hyde SA, Florence J, Gabain AC, Thompson N. Prolongation of walking in Duchenne Muscular Dystrophy with lightweight orthoses; review of 57 cases. Developmental Medicine and Child Neurology 1985;27(2):149‐54. - PubMed
Heckmatt 1989
-
- Heckmatt JZ, Rodillo E, Dubowitz V. Management of children: pharmacological and physical. British Medical Bulletin 1989;45(3):788‐801. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 1987
-
- Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51(6):919‐28. - PubMed
Ishikawa 1995
-
- Ishikawa Y, Bach JR, Ishikawa Y, Minami R. A management trial for Duchenne cardiomyopathy. American Journal of Physical Medicine and Rehabilitation 1995;74(5):345‐50. - PubMed
Ishikawa 1999
-
- Ishikawa Y, Bach JR, Minami R. Cardioprotection for Duchenne's muscular dystrophy. American Heart Journal 1999;137(5):895‐902. - PubMed
Jacobs 1996
-
- Jacobs SC, Bootsma AL, Willems PW, Bar PR, Wokke JH. Prednisolone can protect against exercise‐induced muscle damage. Journal of Neurology 1996;243(5):410‐6. - PubMed
Jeppesen 2003
-
- Jeppesen J, Green A, Steffensen BF, Rahbek J. The Duchenne muscular dystrophy population in Denmark, 1977‐2001: prevalence, incidence and survival in relation to the introduction of ventilator use. Neuromuscular Disorders 2003;13(10):804‐12. - PubMed
Kissel 1991
-
- Kissel JT, Burrow K, Rammohan KW, Mendell JR. Mononuclear cell analysis of muscle biopsies in prednisone‐treated and untreated Duchenne muscular dystrophy. CIDD Study Group. Neurology 1991;41(5):667‐72. - PubMed
Koenig 1989
Matsumura 1993
-
- Matsumura K, Ohlendieck K, Ionasescu VV, Tome FM, Nonaka I, Burghes AH, et al. The role of the dystrophin‐glycoprotein complex in the molecular pathogenesis of muscular dystrophies. Neuromuscular Disorders 1993;3(5):533‐5. - PubMed
Matsumura 1994
-
- Matsumura K, Campbell KP. Dystrophin‐glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle & Nerve 1994;17(1):2‐15. - PubMed
Mendell 1995
-
- Mendell JR, Sahenk Z, Prior TW. The childhood muscular dystrophies: diseases sharing a common pathogenesis of membrane instability. Journal of Child Neurology 1995;10(2):150‐9. - PubMed
Mendell 2012
-
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al‐Dahhak R, Gastier‐Foster J, et al. Evidence‐based path to newborn screening for Duchenne muscular dystrophy. Annals of Neurology 2012;71(3):304–13. - PubMed
Metzinger 1995
Moher 2009
Morrow 2013
Moxley 2005
-
- Moxley RT 3rd, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, et al. Practice Parameter: corticosteroid treatment of Duchenne dystrophy, report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2005;64(1):13‐20. - PubMed
MRC 1976
-
- Medical Research Council. Aids to the Investigation of Peripheral Nerve Injuries. London: HMSO, 1976.
Muntoni 2002
-
- Muntoni F, Fisher I, Morgan JE, Abraham D. Steroids in Duchenne muscular dystrophy: from clinical trials to genomic research. Neuromuscular Disorders 2002;12(Suppl 1):S162‐5. - PubMed
Muntoni 2003
-
- Muntoni F. Cardiomyopathy in muscular dystrophies. Current Opinion in Neurology 2003;16(5):577‐83. - PubMed
Muntoni 2006
-
- Muntoni F, Bushby K, Manzur AY. Muscular Dystrophy Campaign Funded Workshop on Management of Scoliosis in Duchenne Muscular Dystrophy 24 January 2005, London, UK. Neuromuscular Disorders 2006;16(3):210–9. - PubMed
Noguchi 2003
-
- Noguchi S, Tsukahara T, Fujita M, Kurokawa R, Tachikawa M, Toda T, et al. cDNA microarray analysis of individual Duchenne muscular dystrophy patients. Human Molecular Genetics 2003;12(6):595‐600. - PubMed
Pasquini 1995
-
- Pasquini F, Guerin C, Blake D, Davies K, Karpati G, Holland P. The effect of glucocorticoids on the accumulation of utrophin by cultured normal and dystrophic human skeletal muscle satellite cells. Neuromuscular Disorders 1995;5(2):105‐14. - PubMed
Passaquin 1998
Pescatori 2007
-
- Pescatori M, Broccolini A, Minetti C, Bertini E, Bruno C, D'amico A. Gene expression profiling in the early phases of DMD: a constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. The FASEB Journal 2007;21(4):1210‐26. - PubMed
Petrof 1993
Petrof 1998
-
- Petrof BJ. The molecular basis of activity‐induced muscle injury in Duchenne muscular dystrophy. Molecular Cell Biochemistry 1998;179(1‐2):111‐23. [MEDLINE: ] - PubMed
Porter 2002
-
- Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, et al. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin‐deficient mdx mice. Human Molecular Genetics 2002;11(3):263‐72. - PubMed
Porter 2003
-
- Porter JD, Merriam AP, Leahy P, Gong B, Khanna S. Dissection of temporal gene expression signatures of affected and spared muscle groups in dystrophin‐deficient (mdx) mice. Human Molecular Genetics 2003;12(15):1813‐21. - PubMed
Quinlivan 2005
-
- Quinlivan R, Roper H, Davie M, Shaw NJ, McDonagh J, Bushby K. Report of a Muscular Dystrophy Campaign funded workshop Birmingham, UK, January 16th 2004. Osteoporosis in Duchenne muscular dystrophy; its prevalence, treatment and prevention. Neuromuscular Disorders 2005;15(1):72‐9. - PubMed
Quinlivan 2012
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ricotti 2016
Rifai 1995
-
- Rifai Z, Welle E, Moxley RT 3rd, Lorenson M, Griggs RC. Effect of prednisone on protein metabolism in Duchenne muscular dystrophy. American Journal of Physiology 1995;268(1 pt 1):E67‐E74. - PubMed
Rodillo 1988
-
- Rodillo EB, Fernandez‐Bermejo E, Heckmatt JZ, Dubowitz V. Prevention of rapidly progressive scoliosis in Duchenne muscular dystrophy by prolongation of walking with orthoses. Journal of Child Neurology 1988;3(4):269‐74. - PubMed
Scott 1982
-
- Scott OM, Hyde SA, Goddard C, Dubowitz V. Quantitation of muscle function in children: a prospective study in Duchenne muscular dystrophy. Muscle & Nerve 1982;5(4):291‐301. - PubMed
Scott 2012
-
- Scott E, Eagle M, Mayhew A, Freeman J, Main M, Sheehan J, et al. North Star Clinical Network for Paediatric Neuromuscular Disease. Development of a functional assessment scale for ambulatory boys with Duchenne muscular dystrophy. Physiotherapy Research International 2012;17(2):101‐9. - PubMed
Sharma 1993
-
- Sharma KR, Mynhier MA, Miller RG. Cyclosporine increases muscular force generation in Duchenne muscular dystrophy. Neurology 1993;43(3):527‐32. - PubMed
Spencer 1962
-
- Spencer GE, Vignos PJ. Bracing for ambulation in childhood progressive muscular dystrophy. Journal of Bone and Joint Surgery 1962;44A:234‐42. - PubMed
Vandebrouck 1999
-
- Vandebrouck C, Imbert N, Dupont G, Cognard C, Raymond G. The effect of methylprednisone on intracellular calcium of normal and dystrophic human skeletal muscle cells. Neuroscience Letters 1999;269(2):110‐4. - PubMed
References to other published versions of this review
Manzur 2002
-
- Manzur AY, Pike M, Elliot T. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003725.pub2] - DOI
Manzur 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

