The Kinetics of Circulating Monocyte Subsets and Monocyte-Platelet Aggregates in the Acute Phase of ST-Elevation Myocardial Infarction: Associations with 2-Year Cardiovascular Events
- PMID: 27149446
- PMCID: PMC4863763
- DOI: 10.1097/MD.0000000000003466
The Kinetics of Circulating Monocyte Subsets and Monocyte-Platelet Aggregates in the Acute Phase of ST-Elevation Myocardial Infarction: Associations with 2-Year Cardiovascular Events
Abstract
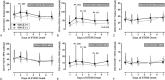
In experimental myocardial infarction (MI), a rise in cell counts of circulating monocyte subsets contributes to impaired myocardial healing and to atherosclerotic plaque destabilization. In humans, the prognostic role of monocyte subsets in patients suffering ST-elevation MI (STEMI) is still unclear. In the present study, we aimed to determine the kinetics of the 3 monocyte subsets (classical CD14++CD16-, intermediate CD14++CD16+, and nonclassical CD14+CD16++ monocytes), as well as the subset-specific monocyte-platelet aggregates (MPA), in acute STEMI followed by primary percutaneous coronary intervention (PCI), and their relationships with cardiovascular outcomes during a 2-year follow-up.Monocyte subsets and MPA were measured in 100 STEMI patients receiving primary PCI on days 1, 2, 3, 5, and 7 of symptom onset, which were compared with 60 stable coronary heart disease patients and 35 healthy volunteers. From day 1 to day 7, significant increases in the counts of CD14++CD16+ monocytes and CD14++CD16+ MPA were observed, with peak levels on day 2. During a median follow-up of 2.0 years, 28 first cardiovascular events (defined as cardiovascular death, nonfatal ischemic stroke, recurrent MI, need for emergency or repeat revascularization, and rehospitalization for heart failure) were recorded. After adjustment for confounders, CD14++CD16+ monocytosis (day 1 [HR: 3.428; 95% CI: 1.597-7.358; P = 0.002], day 2 [HR: 4.835; 95% CI: 1.106-21.13; P = 0.04], day 3 [HR: 2.734; 95% CI: 1.138-6.564; P = 0.02], and day 7 [HR: 2.647; 95% CI: 1.196-5.861; P = 0.02]), as well as increased levels of CD14++CD16+ MPA measured on all time points (days 1, 2, 3, 5, and 7), had predictive values for adverse cardiovascular events.In conclusion, our data show the expansion of the CD14++CD16+ monocyte subset during acute phase of STEMI has predictive values for 2-year adverse cardiovascular outcomes in patients treated with primary PCI. Future studies will be warranted to elucidate whether CD14++CD16+ monocytes may become a target cell population for new therapeutic strategies after STEMI.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures




References
-
- Seropian IM, Toldo S, Van Tassell BW, et al. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol 2014; 63:1593–1603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

