Sensitization of the Nociceptive System in Complex Regional Pain Syndrome
- PMID: 27149519
- PMCID: PMC4858201
- DOI: 10.1371/journal.pone.0154553
Sensitization of the Nociceptive System in Complex Regional Pain Syndrome
Abstract
Background: Complex regional pain syndrome type I (CRPS-I) is characterized by sensory, motor and autonomic abnormalities without electrophysiological evidence of a nerve lesion.
Objective: Aims were to investigate how sensory, autonomic and motor function change in the course of the disease.
Methods: 19 CRPS-I patients (17 with acute, 2 with chronic CRPS, mean duration of disease 5.7±8.3, range 1-33 months) were examined with questionnaires (LANSS, NPS, MPI, Quick DASH, multiple choice list of descriptors for sensory, motor, autonomic symptoms), motor and autonomic tests as well as quantitative sensory testing according to the German Research Network on Neuropathic Pain at two visits (baseline and 36±10.6, range 16-53 months later).
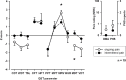
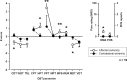
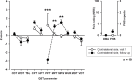
Results: CRPS-I patients had an improvement of sudomotor and vasomotor function, but still a great impairment of sensory and motor function upon follow-up. Although pain and mechanical detection improved upon follow-up, thermal and mechanical pain sensitivity increased, including the contralateral side. Increase in mechanical pain sensitivity and loss of mechanical detection were associated with presence of ongoing pain.
Conclusions: The results demonstrate that patients with CRPS-I show a sensitization of the nociceptive system in the course of the disease, for which ongoing pain seems to be the most important trigger. They further suggest that measured loss of function in CRPS-I is due to pain-induced hypoesthesia rather than a minimal nerve lesion. In conclusion, this article gives evidence for a pronociceptive pain modulation profile developing in the course of CRPS and thus helps to assess underlying mechanisms of CRPS that contribute to the maintenance of patients' pain and disability.
Conflict of interest statement
Figures

References
-
- Harden N, Bruehl S. Diagnostic criteria: The statistical derivation of the four criterion factors In: Wilson P, Stanton-Hicks M, Harden N, editors. CRPS: Current diagnosis and therapy. Seattle: IASP Press; 2005. p. 45–58.
-
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

