A Mutation in LTBP2 Causes Congenital Glaucoma in Domestic Cats (Felis catus)
- PMID: 27149523
- PMCID: PMC4858209
- DOI: 10.1371/journal.pone.0154412
A Mutation in LTBP2 Causes Congenital Glaucoma in Domestic Cats (Felis catus)
Erratum in
-
Correction: A Mutation in LTBP2 Causes Congenital Glaucoma in Domestic Cats (Felis catus).PLoS One. 2016 Aug 18;11(8):e0161517. doi: 10.1371/journal.pone.0161517. eCollection 2016. PLoS One. 2016. PMID: 27537365 Free PMC article.
Abstract
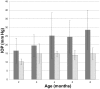
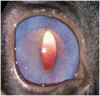
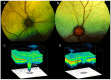
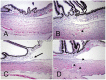
The glaucomas are a group of diseases characterized by optic nerve damage that together represent a leading cause of blindness in the human population and in domestic animals. Here we report a mutation in LTBP2 that causes primary congenital glaucoma (PCG) in domestic cats. We identified a spontaneous form of PCG in cats and established a breeding colony segregating for PCG consistent with fully penetrant, autosomal recessive inheritance of the trait. Elevated intraocular pressure, globe enlargement and elongated ciliary processes were consistently observed in all affected cats by 8 weeks of age. Varying degrees of optic nerve damage resulted by 6 months of age. Although subtle lens zonular instability was a common feature in this cohort, pronounced ectopia lentis was identified in less than 10% of cats examined. Thus, glaucoma in this pedigree is attributed to histologically confirmed arrest in the early post-natal development of the aqueous humor outflow pathways in the anterior segment of the eyes of affected animals. Using a candidate gene approach, significant linkage was established on cat chromosome B3 (LOD 18.38, θ = 0.00) using tightly linked short tandem repeat (STR) loci to the candidate gene, LTBP2. A 4 base-pair insertion was identified in exon 8 of LTBP2 in affected individuals that generates a frame shift that completely alters the downstream open reading frame and eliminates functional domains. Thus, we describe the first spontaneous and highly penetrant non-rodent model of PCG identifying a valuable animal model for primary glaucoma that closely resembles the human disease, providing valuable insights into mechanisms underlying the disease and a valuable animal model for testing therapies.
Conflict of interest statement
Figures

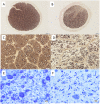
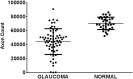
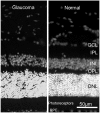
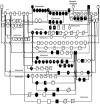


References
-
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014. June 26 . - PubMed
-
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Archives of Ophthalmology. 2003. January;121(1):48–56. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

