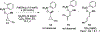Photoinduced Formation of Hybrid Aryl Pd-Radical Species Capable of 1,5-HAT: Selective Catalytic Oxidation of Silyl Ethers into Silyl Enol Ethers
- PMID: 27149524
- PMCID: PMC7577783
- DOI: 10.1021/jacs.6b01628
Photoinduced Formation of Hybrid Aryl Pd-Radical Species Capable of 1,5-HAT: Selective Catalytic Oxidation of Silyl Ethers into Silyl Enol Ethers
Abstract
A direct visible light-induced generation of a hybrid aryl Pd-radical species from aryl iodide and Pd(0) is reported to enable an unprecedented (for hybrid Pd-radical species) hydrogen atom-transfer event. This approach allowed for efficient desaturation of readily available silyl ethers into synthetically valuable silyl enols. Moreover, this oxidation reaction proceeds at room temperature without the aid of exogenous photosensitizers or oxidants.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:
Experimental details and data (PDF)
Figures
Similar articles
-
alpha-Nitration of Ketones via Enol Silyl Ethers. Radical Cations as Reactive Intermediates in Thermal and Photochemical Processes.J Org Chem. 1996 Jan 26;61(2):627-639. doi: 10.1021/jo9515687. J Org Chem. 1996. PMID: 11666984
-
Alkoxy radical cyclizations onto silyl enol ethers relative to alkene cyclization, hydrogen atom transfer, and fragmentation reactions.J Org Chem. 2011 Oct 7;76(19):7720-9. doi: 10.1021/jo200992m. Epub 2011 Aug 26. J Org Chem. 2011. PMID: 21819036
-
Expanding the chemical space of enol silyl ethers: catalytic dicarbofunctionalization enabled by iron catalysis.Chem Sci. 2023 Oct 20;14(45):13007-13013. doi: 10.1039/d3sc04549h. eCollection 2023 Nov 22. Chem Sci. 2023. PMID: 38023494 Free PMC article.
-
General, Mild, and Selective Method for Desaturation of Aliphatic Amines.J Am Chem Soc. 2018 Feb 21;140(7):2465-2468. doi: 10.1021/jacs.8b00488. Epub 2018 Feb 7. J Am Chem Soc. 2018. PMID: 29400959 Free PMC article.
-
Direct allylic C-H alkylation of enol silyl ethers enabled by photoredox-Brønsted base hybrid catalysis.Nat Commun. 2019 Jun 20;10(1):2706. doi: 10.1038/s41467-019-10641-y. Nat Commun. 2019. PMID: 31221955 Free PMC article.
Cited by
-
Catalysis with Palladium Complexes Photoexcited by Visible Light.Angew Chem Int Ed Engl. 2019 Aug 19;58(34):11586-11598. doi: 10.1002/anie.201813523. Epub 2019 May 24. Angew Chem Int Ed Engl. 2019. PMID: 30600875 Free PMC article. Review.
-
Aryl-to-alkyl radical relay Heck reaction of amides with vinyl arenes.Chem Sci. 2023 Feb 27;14(13):3580-3586. doi: 10.1039/d2sc06852d. eCollection 2023 Mar 29. Chem Sci. 2023. PMID: 37006700 Free PMC article.
-
Transition-metal-free C(sp3)-H/C(sp3)-H dehydrogenative coupling of saturated heterocycles with N-benzyl imines.Chem Sci. 2020 Mar 31;11(29):7619-7625. doi: 10.1039/d0sc00031k. Chem Sci. 2020. PMID: 34094139 Free PMC article.
-
Visible-Light-Driven C-S Bond Formation Based on Electron Donor-Acceptor Excitation and Hydrogen Atom Transfer Combined System.ACS Org Inorg Au. 2021 Jun 22;1(1):23-28. doi: 10.1021/acsorginorgau.1c00007. eCollection 2021 Oct 6. ACS Org Inorg Au. 2021. PMID: 36855634 Free PMC article.
-
Heck Reaction of Electronically Diverse Tertiary Alkyl Halides.Org Lett. 2018 Jan 19;20(2):357-360. doi: 10.1021/acs.orglett.7b03591. Epub 2018 Jan 5. Org Lett. 2018. PMID: 29303271 Free PMC article.
References
-
- For selected reviews on Pd-catalyzed reactions, see: Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi E; John Wiley & Sons: West Sussex, U.K., 2003.
- In Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; de Meijere A, Diederich F; Eds.; Wiley-VCH: Weinheim, Germany, 2004, 815.
- Selander N; Szabó KJ Chem. Rev 2011, 111, 2048. - PubMed
- Molnar A Chem. Rev 2011, 111, 2251. - PubMed
- Knappke CEI; Wangelin AJ Chem. Soc. Rev 2011, 40, 4948. - PubMed
- Seechun CJ; Kitching MO; Colacot TJ; Snieckus V Angew. Chem., Int. Ed 2012, 51, 5062. - PubMed
-
- Recently, a plethora of methods involving alkyl hybrid-Pd radical intermediates have been reported, most of which have led to unique reactivity and selectivity due to the intrinsic characteristics of the hybrid intermediates. However, direct formation and utilization of aryl hybrid-Pd radical intermediates has not been reported. For a recent review on Pd involved radical reactions, see: Liu Q; Dong X; Li J; Xiao J; Dong Y; Liu H ACS Catal 2015, 5, 6111.
- For a review on catalyzed radical reactions, see: Studer A; Curran DP Angew. Chem., Int. Ed 2016, 55, 58. - PubMed
-
- Curran DP; Kim D; Liu HT; Shen WJ Am. Chem. Soc 1988, 110, 5900.
- For HAT reviews, see: Majetich G; Wheless K Tetrahedron 1995, 51, 7095.
- Radicals in Organic Synthesis; Renaud P, Sibi MP; Wiley-VCH: Weinheim, 2001; Vol. 2.
- Robertson J; Pillai J; Lush RK Chem. Soc. Rev. 2001, 30, 94.
- Nechab M; Mondal S; Bertrand MP Chem. - Eur. J 2014, 20, 16034. - PubMed
-
- For an impressive example on photoinduced desaturation reaction involving HAT process, see: Breslow R; Baldwin S; Flechtner T; Kalicky P; Liu S; Washburn W J. Am. Chem. Soc 1973, 95, 3251. - PubMed
- For efficient desaturation reactions involving both free radical and cationic intermediates, see: Voica A; Mendoza A; Gutekunst WR; Fraga JO; Baran PS Nat. Chem 2012, 4, 629. - PMC - PubMed
- Hollister KA; Conner ES; Spell ML; Deveaux K; Maneval L; Beal MW; Ragains JR Angew. Chem., Int. Ed 2015, 54, 7837. - PubMed
-
- For desilylative oxidation of silyl ethers into carbonyl derivatives, see: Reddy MS; Narender M; Nageswar YVD; Rao KR Synthesis 2005, 2005, 714.
- Chandrasekhar S; Mohanty PK; Takhi MJ Org. Chem. 1997, 62, 2628. - PubMed
- Liu H-J; Han I-S Synth. Commun. 1985, 15, 759.
- Olah G; Balaram Gupta GB Synthesis 1980, 1980, 44.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources