Improved Monosynaptic Neural Circuit Tracing Using Engineered Rabies Virus Glycoproteins
- PMID: 27149846
- PMCID: PMC5063660
- DOI: 10.1016/j.celrep.2016.03.067
Improved Monosynaptic Neural Circuit Tracing Using Engineered Rabies Virus Glycoproteins
Abstract
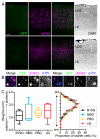
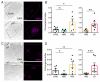
Monosynaptic rabies virus tracing is a unique and powerful tool used to identify neurons making direct presynaptic connections onto neurons of interest across the entire nervous system. Current methods utilize complementation of glycoprotein gene-deleted rabies of the SAD B19 strain with its glycoprotein, B19G, to mediate retrograde transsynaptic spread across a single synaptic step. In most conditions, this method labels only a fraction of input neurons and would thus benefit from improved efficiency of transsynaptic spread. Here, we report newly engineered glycoprotein variants to improve transsynaptic efficiency. Among them, oG (optimized glycoprotein) is a codon-optimized version of a chimeric glycoprotein consisting of the transmembrane/cytoplasmic domain of B19G and the extracellular domain of rabies Pasteur virus strain glycoprotein. We demonstrate that oG increases the tracing efficiency for long-distance input neurons up to 20-fold compared to B19G. oG-mediated rabies tracing will therefore allow identification and study of more complete monosynaptic input neural networks.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

