Serum microRNA-1233 is a specific biomarker for diagnosing acute pulmonary embolism
- PMID: 27150028
- PMCID: PMC4858885
- DOI: 10.1186/s12967-016-0886-9
Serum microRNA-1233 is a specific biomarker for diagnosing acute pulmonary embolism
Abstract
Background: Circulating microRNAs (miRNAs) emerge as novel biomarkers in cardiovascular diseases. Diagnosing acute pulmonary embolism (PE) remains challenging due to a diverse clinical presentation and the lack of specific biomarkers. Here we evaluate serum miRNAs as potential biomarkers in acute PE.
Methods: We enrolled 30 patients with acute, CT (computed tomography)-angiographically confirmed central PE and collected serum samples on the day of emergency room admission (1st day) and from 22 of these patients 9 months thereafter. For comparison, we examined serum samples from patients with acute non ST-segment elevation myocardial infarction (NSTEMI, n = 30) and healthy individuals (n = 12).
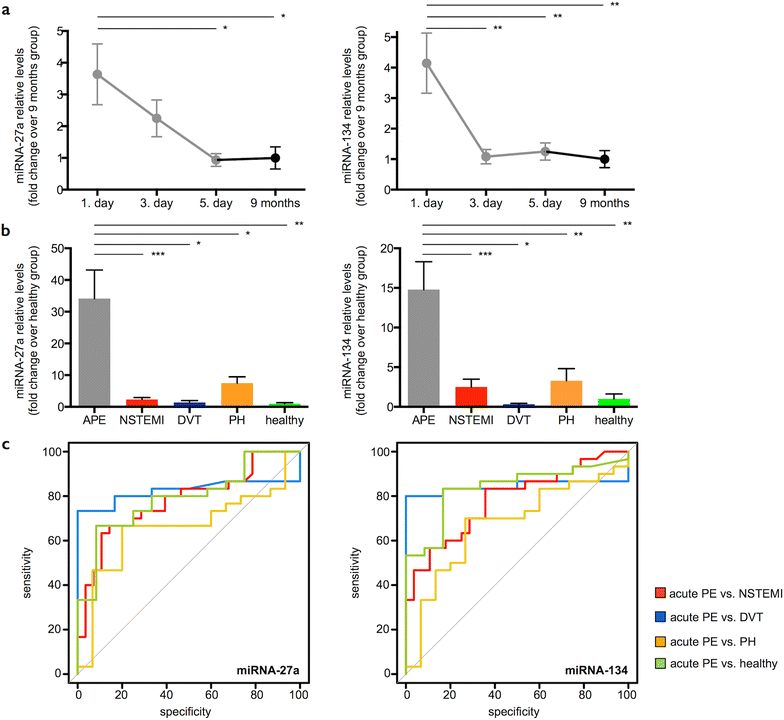
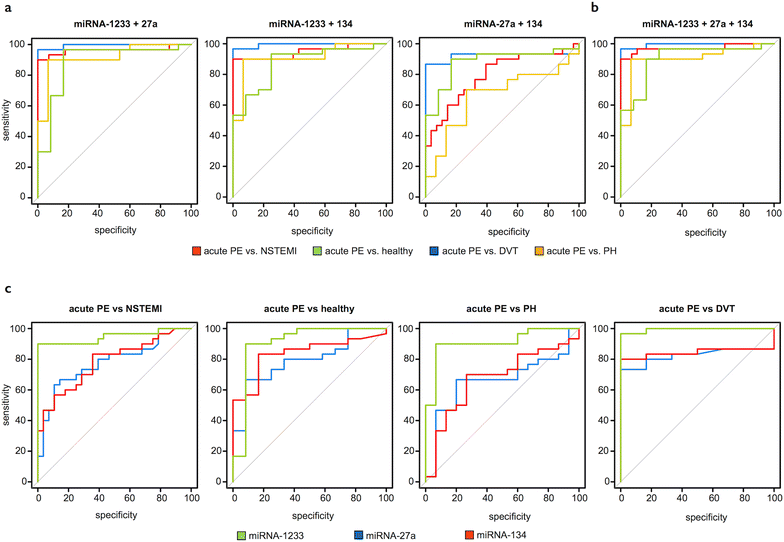
Results: We randomly selected 16 out of 30 PE patients and screened sera from the acute (1st day) and chronic stages (9 months) for 754 miRNAs using microarrays and found 37 miRNAs to be differentially regulated. Across all miRNAs, miRNA-1233 displayed the highest fold change (FC) from acute to chronic stage (log2FC 11.5, p < 0.004). We validated miRNA-1233 by real-time quantitative polymerase chain reaction (RT-qPCR). In acute PE (1st day) we found elevated levels of miRNA-1233 in comparison to NSTEMI (log2FC 5.7, p < 0.0001) and healthy controls (log2FC 7.7, p < 0.0001). miRNA-1233 differentiated acute PE from NSTEMI patients and healthy individuals with 90 and 90 % sensitivity, and 100 and 92 % specificity [area under the curve (AUC) 0.95, p < 0.001 and 0.91, p < 0.001], respectively.
Conclusions: This is the first report that identifies a miRNA that allows distinguishing acute PE from acute NSTEMI and healthy individuals with high specificity and sensitivity.
Keywords: Biomarker; Non ST-segment elevation myocardial infarction micro-RNA; Pulmonary embolism.
Figures


References
-
- Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

