Analysis of random PCR-originated mutants of the yeast Ste2 and Ste3 receptors
- PMID: 27150158
- PMCID: PMC4985600
- DOI: 10.1002/mbo3.361
Analysis of random PCR-originated mutants of the yeast Ste2 and Ste3 receptors
Abstract
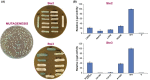
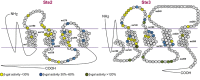
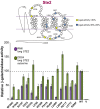
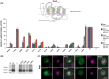
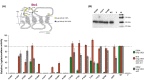
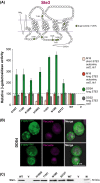
The G protein-coupled receptors Ste2 and Ste3 bind α- and a-factor, respectively, in Saccharomyces cerevisiae. These receptors share a similar conformation, with seven transmembrane segments, three intracellular loops, a C-terminus tail, and three extracellular loops. However, the amino acid sequences of these two receptors bear no resemblance to each other. Coincidently the two ligands, α- and a-factor, have different sequences. Both receptors activate the same G protein. To identify amino acid residues that are important for signal transduction, the STE2 and STE3 genes were mutagenized by a random PCR-based method. Mutant receptors were analyzed in MATα cells mutated in the ITC1 gene, whose product represses transcription of a-specific genes in MATα. Expression of STE2 or STE3 in these cells results in autocrine activation of the mating pathway, since this strain produces the Ste2 receptor in addition to its specific ligand, α-factor. It also produces a-factor in addition to its specific receptor, Ste3. Therefore, this strain provides a convenient model to analyze mutants of both receptors in the same background. Many hyperactive mutations were found in STE3, whereas none was detected in STE2. This result is consistent with the different strategies that the two genes have adopted to be expressed.
Keywords: G protein-coupled receptors; hyperactive mutations; mating response; signal transduction.
© 2016 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Figures
References
-
- Apanovitch, D. M. , Slep K. C., Sigler P. B., and Dohlman H. G.. 1998. Sst2 is a GTPase‐activating protein for Gpa1: purification and characterization of a cognate RGS‐Galpha protein pair in yeast. Biochemistry 37:4815–4822. - PubMed
-
- Ballon, D. R. , Flanary P. L., Gladue D. P., Konopka J. B., Dohlman H. G., and Thorner J.. 2006. DEP‐domain‐mediated regulation of GPCR signaling responses. Cell 126:1079–1093. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

