Cysteamine broadly improves the anti-plasmodial activity of artemisinins against murine blood stage and cerebral malaria
- PMID: 27150250
- PMCID: PMC4858922
- DOI: 10.1186/s12936-016-1317-3
Cysteamine broadly improves the anti-plasmodial activity of artemisinins against murine blood stage and cerebral malaria
Abstract
Background: The potential emergence and spread of resistance to artemisinins in the Plasmodium falciparum malaria parasite constitutes a major global health threat. Hence, improving the efficacy of artemisinins and of artemisinin-based combination therapy (ACT) represents a major short-term goal in the global fight against malaria. Mice defective in the enzyme pantetheinase (Vnn3) show increased susceptibility to blood-stage malaria (increased parasitaemia, reduced survival), and supplementation of Vnn3 mutants with the reaction product of pantetheinase, cysteamine, corrects in part the malaria-susceptibility phenotype of the mutants. Cysteamine (Cys) is a small, naturally occurring amino-thiol that has very low toxicity in vivo and is approved for clinical use in the life-long treatment of the kidney disorder nephropathic cystinosis.
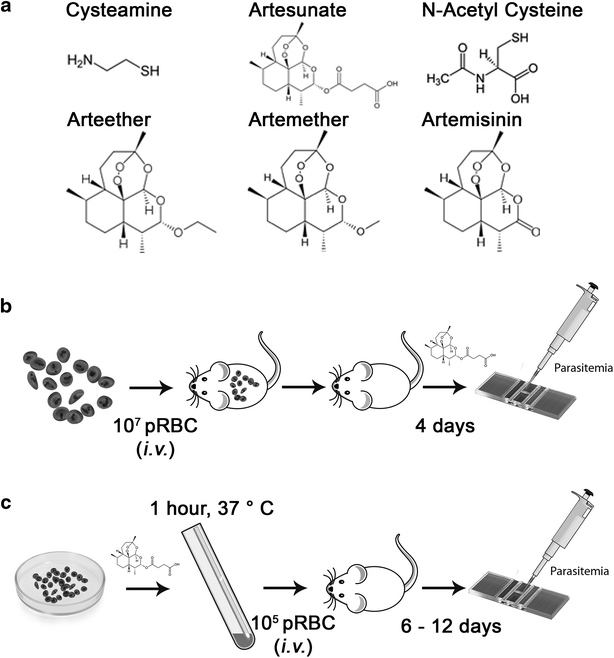
Methods: The ability of Cys to improve the anti-plasmodial activity of different clinically used artemisinins was tested. The effect of different CYS/ART combinations on malarial phenotypes (parasite blood-stage replication, overall and survival from lethal infection) was assessed in a series of in vivo experiments using Plasmodium strains that induce either blood-stage (Plasmodium chabaudi AS) or cerebral disease (Plasmodium berghei ANKA). This was also evaluated in an ex vivo experimental protocol that directly assesses the effect of such drug combinations on the viability of Plasmodium parasites, as measured by the ability of tested parasites to induce a productive infection in vivo in otherwise naïve animals.
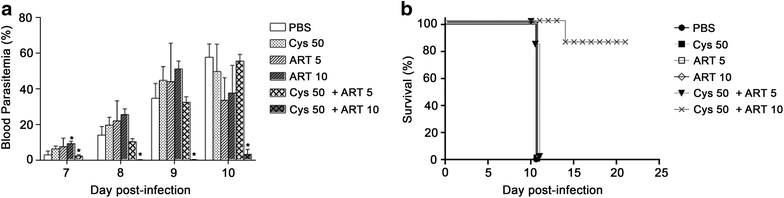
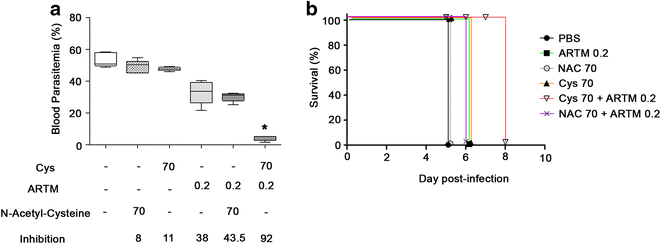
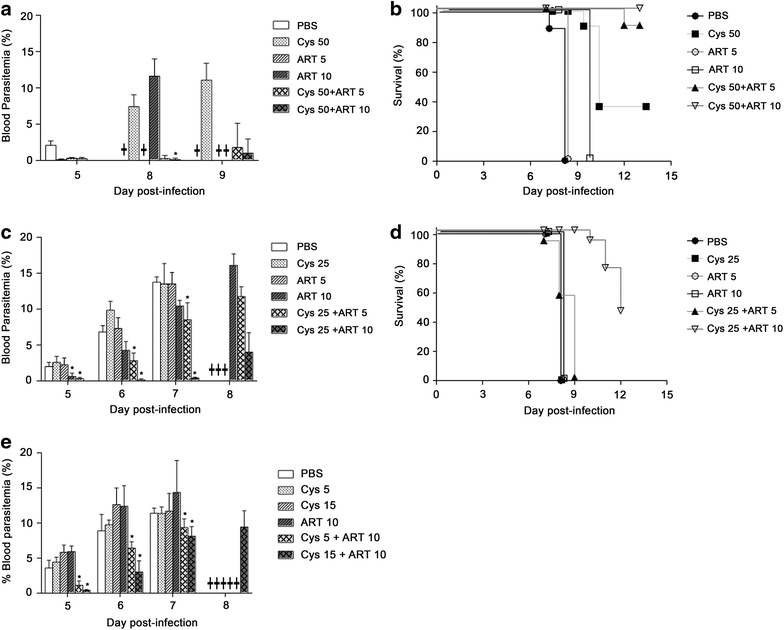
Results: Cys is found to potentiate the anti-plasmodial activity of artesunate, artemether, and arteether, towards the blood-stage malaria parasite P. chabaudi AS. Ex vivo experiments, indicate that potentiation of the anti-plasmodial activity of artemisinins by Cys is direct and does not require the presence of host factors. In addition, potentiation occurs at sub-optimal concentrations of artemisinins and Cys that on their own have little or no effect on parasite growth. Cys also dramatically enhances the efficacy and protective effect of artemisinins against cerebral malaria induced by infection with the P. berghei ANKA parasite.
Conclusion: These findings indicate that inclusion of Cys in current formulations of ACT, or its use as adjunct therapy could improve the anti-plasmodial activity of artemisinin, decrease mortality in cerebral malaria patients, and prevent or delay the development and spread of artemisinin resistance.
Keywords: Artemisinins; Blood-stage malaria; Cerebral malaria; Cysteamine; Drug resistance; Plasmodium.
Figures


Similar articles
-
Cysteamine, the molecule used to treat cystinosis, potentiates the antimalarial efficacy of artemisinin.Antimicrob Agents Chemother. 2010 Aug;54(8):3262-70. doi: 10.1128/AAC.01719-09. Epub 2010 May 17. Antimicrob Agents Chemother. 2010. PMID: 20479197 Free PMC article.
-
Genetic analysis in mice identifies cysteamine as a novel partner for artemisinin in the treatment of malaria.Mamm Genome. 2011 Aug;22(7-8):486-94. doi: 10.1007/s00335-011-9316-8. Epub 2011 Mar 25. Mamm Genome. 2011. PMID: 21437649 Review.
-
Treatment of murine cerebral malaria by artemisone in combination with conventional antimalarial drugs: antiplasmodial effects and immune responses.Antimicrob Agents Chemother. 2014 Aug;58(8):4745-54. doi: 10.1128/AAC.01553-13. Epub 2014 Jun 9. Antimicrob Agents Chemother. 2014. PMID: 24913162 Free PMC article.
-
In vitro and in vivo anti-malarial activity of novel harmine-analog heat shock protein 90 inhibitors: a possible partner for artemisinin.Malar J. 2016 Dec 1;15(1):579. doi: 10.1186/s12936-016-1625-7. Malar J. 2016. PMID: 27903279 Free PMC article.
-
Current scenario and future strategies to fight artemisinin resistance.Parasitol Res. 2019 Jan;118(1):29-42. doi: 10.1007/s00436-018-6126-x. Epub 2018 Nov 26. Parasitol Res. 2019. PMID: 30478733 Review.
Cited by
-
Elimination of Hepatic Rodent Plasmodium Parasites by Amino Acid Supplementation.iScience. 2020 Nov 6;23(12):101781. doi: 10.1016/j.isci.2020.101781. eCollection 2020 Dec 18. iScience. 2020. PMID: 33294789 Free PMC article.
-
Rocaglates as dual-targeting agents for experimental cerebral malaria.Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):E2366-E2375. doi: 10.1073/pnas.1713000115. Epub 2018 Feb 20. Proc Natl Acad Sci U S A. 2018. PMID: 29463745 Free PMC article.
-
Immunomodulatory effects of cysteamine and its potential use as a host-directed therapy for tuberculosis.Front Immunol. 2024 Oct 28;15:1411827. doi: 10.3389/fimmu.2024.1411827. eCollection 2024. Front Immunol. 2024. PMID: 39530101 Free PMC article.
-
Cysteamine, an Endogenous Aminothiol, and Cystamine, the Disulfide Product of Oxidation, Increase Pseudomonas aeruginosa Sensitivity to Reactive Oxygen and Nitrogen Species and Potentiate Therapeutic Antibiotics against Bacterial Infection.Infect Immun. 2018 May 22;86(6):e00947-17. doi: 10.1128/IAI.00947-17. Print 2018 Jun. Infect Immun. 2018. PMID: 29581193 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

