The Pharmacogenomics of Bipolar Disorder study (PGBD): identification of genes for lithium response in a prospective sample
- PMID: 27150464
- PMCID: PMC4857276
- DOI: 10.1186/s12888-016-0732-x
The Pharmacogenomics of Bipolar Disorder study (PGBD): identification of genes for lithium response in a prospective sample
Abstract
Background: Bipolar disorder is a serious and common psychiatric disorder characterized by manic and depressive mood switches and a relapsing and remitting course. The cornerstone of clinical management is stabilization and prophylaxis using mood-stabilizing medications to reduce both manic and depressive symptoms. Lithium remains the gold standard of treatment with the strongest data for both efficacy and suicide prevention. However, many patients do not respond to this medication, and clinically there is a great need for tools to aid the clinician in selecting the correct treatment. Large genome wide association studies (GWAS) investigating retrospectively the effect of lithium response are in the pipeline; however, few large prospective studies on genetic predictors to of lithium response have yet been conducted. The purpose of this project is to identify genes that are associated with lithium response in a large prospective cohort of bipolar patients and to better understand the mechanism of action of lithium and the variation in the genome that influences clinical response.
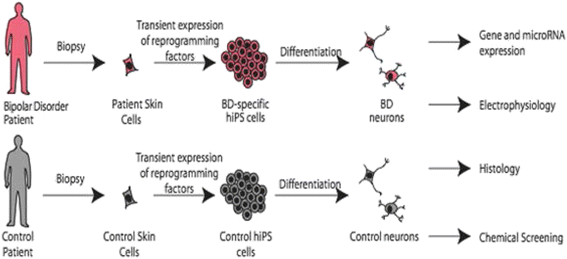
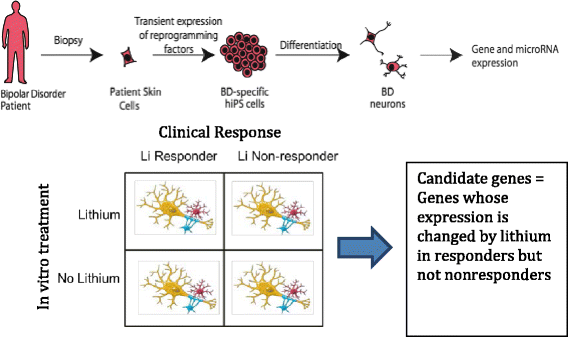
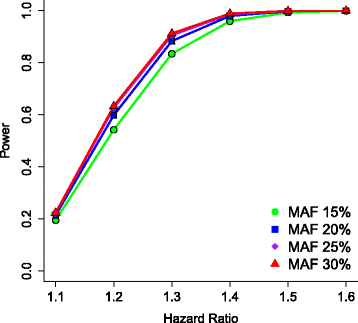
Methods/design: This study is an 11-site prospective non-randomized open trial of lithium designed to ascertain a cohort of 700 subjects with bipolar I disorder who experience protocol-defined relapse prevention as a result of treatment with lithium monotherapy. All patients will be diagnosed using the Diagnostic Interview for Genetic Studies (DIGS) and will then enter a 2-year follow-up period on lithium monotherapy if and when they exhibit a score of 1 (normal, not ill), 2 (minimally ill) or 3 (mildly ill) on the Clinical Global Impressions of Severity Scale for Bipolar Disorder (CGI-S-BP Overall Bipolar Illness) for 4 of the 5 preceding weeks. Lithium will be titrated as clinically appropriate, not to exceed serum levels of 1.2 mEq/L. The sample will be evaluated longitudinally using a wide range of clinical scales, cognitive assessments and laboratory tests. On relapse, patients will be discontinued or crossed-over to treatment with valproic acid (VPA) or treatment as usual (TAU). Relapse is defined as a DSM-IV manic, major depressive or mixed episode or if the treating physician decides a change in medication is clinically necessary. The sample will be genotyped for GWAS. The outcome for lithium response will be analyzed as a time to event, where the event is defined as clinical relapse, using a Cox Proportional Hazards model. Positive single nucleotide polymorphisms (SNPs) from past genetic retrospective studies of lithium response, the Consortium on Lithium Genetics (ConLiGen), will be tested in this prospective study sample; a meta-analysis of these samples will then be performed. Finally, neurons will be derived from pluripotent stem cells from lithium responders and non-responders and tested in vivo for response to lithium by gene expression studies. SNPs in genes identified in these cellular studies will also be tested for association to response.
Discussion: Lithium is an extraordinarily important therapeutic drug in the clinical management of patients suffering from bipolar disorder. However, a significant proportion of patients, 30-40 %, fail to respond, and there is currently no method to identify the good lithium responders before initiation of treatment. Converging evidence suggests that genetic factors play a strong role in the variation of response to lithium, but only a few genes have been tested and the samples have largely been retrospective or quite small. The current study will collect an entirely unique sample of 700 patients with bipolar disorder to be stabilized on lithium monotherapy and followed for up to 2 years. This study will produce useful information to improve the understanding of the mechanism of action of lithium and will add to the development of a method to predict individual response to lithium, thereby accelerating recovery and reducing suffering and cost.
Trial registration: ClinicalTrials.gov Identifier: NCT01272531 Registered: January 6, 2011.
Keywords: Bipolar disorder; GWAS; Lithium; Mood stabilizer; Personalized medicine; Pharmacogenetics; Precision medicine; Prospective trial.
Figures
References
-
- Nierenberg AA, Friedman ES, Bowden CL, Sylvia LG, Thase ME, Ketter T, et al. Lithium treatment moderate-dose use study (LiTMUS) for bipolar disorder: a randomized comparative effectiveness trial of optimized personalized treatment with and without lithium. Am J Psychiatry. 2013;170(1):102–10. doi: 10.1176/appi.ajp.2012.12060751. - DOI - PubMed
-
- Mitchell PB, Hadzi-Pavlovic D. John Cade and the discovery of lithium treatment for manic depressive illness. Med J Aust. 1999;171(5):262–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

