High-density lipoprotein, mitochondrial dysfunction and cell survival mechanisms
- PMID: 27150975
- PMCID: PMC4972637
- DOI: 10.1016/j.chemphyslip.2016.04.007
High-density lipoprotein, mitochondrial dysfunction and cell survival mechanisms
Abstract
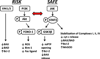
Ischemic injury is associated with acute myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting and open heart surgery. The timely re-establishment of blood flow is critical in order to minimize cardiac complications. Reperfusion after a prolonged ischemic period, however, can induce severe cardiomyocyte dysfunction with mitochondria serving as a major target of ischemia/reperfusion (I/R) injury. An increase in the formation of reactive oxygen species (ROS) induces damage to mitochondrial respiratory complexes leading to uncoupling of oxidative phosphorylation. Mitochondrial membrane perturbations also contribute to calcium overload, opening of the mitochondrial permeability transition pore (mPTP) and the release of apoptotic mediators into the cytoplasm. Clinical and experimental studies show that ischemic preconditioning (ICPRE) and postconditioning (ICPOST) attenuate mitochondrial injury and improve cardiac function in the context of I/R injury. This is achieved by the activation of two principal cell survival cascades: 1) the Reperfusion Injury Salvage Kinase (RISK) pathway; and 2) the Survivor Activating Factor Enhancement (SAFE) pathway. Recent data suggest that high density lipoprotein (HDL) mimics the effects of conditioning protocols and attenuates myocardial I/R injury via activation of the RISK and SAFE signaling cascades. In this review, we discuss the roles of apolipoproteinA-I (apoA-I), the major protein constituent of HDL, and sphingosine 1-phosphate (S1P), a lysosphingolipid associated with small, dense HDL particles as mediators of cardiomyocyte survival. Both apoA-I and S1P exert an infarct-sparing effect by preventing ROS-dependent injury and inhibiting the opening of the mPTP.
Keywords: ApoA-I; Hdl; Ischemia-reperfusion; Mitochondrion; Myocardium; Sphingosine 1-Phosphate.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures
References
-
- Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. - PubMed
-
- Fazio S, Linton MF. Sorting out the complexities of reverse cholesterol transport: CETP polymorphisms, HDL, and coronary disease. J Clin Endocrinol Metab. 2006;91:3273–3275. - PubMed
-
- Ansell BJ, Watson KE, Fogelman AM, Navab M, Fonarow GC. High-density lipoprotein function: recent advances. J Am Coll Cardiol. 2005;46:1792–1798. - PubMed
-
- Assmann G, Nofer JR. Atheroprotective effects of high-density lipoproteins. Annu Rev Med. 2003;54:321–341. - PubMed
-
- Dunbar RL, Rader DJ. Current drug options for raising HDL cholesterol. Curr Treat Options Cardiovasc Med. 2005;7:15–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

