Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice
- PMID: 27151180
- PMCID: PMC4955750
- DOI: 10.1016/j.jhep.2016.04.025
Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice
Abstract
Background & aims: Acetaminophen (APAP)-induced liver injury is the most frequent cause of acute liver failure in the US and many other countries. Metabolism of APAP results in formation of APAP protein adducts (APAP-AD) in hepatocytes and triggers mitochondrial dysfunction and necrosis. However, the mechanisms for how APAP-AD are removed from hepatocytes remain unknown.
Methods: Mice or primary hepatocytes were treated with APAP. APAP-AD were determined by immunoblot, immunostaining and high pressure liquid chomatography with electrochemical detection analysis.
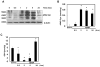
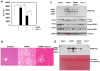
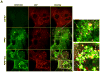
Results: We found that APAP-AD were detected at 1h, peaked at approximately 2h, declined at 6h and almost full removed at 24h post treatment with APAP in mouse livers and in primary mouse hepatocytes. APAP-AD displayed a punctate pattern and were colocalized with GFP-LC3 positive autophagosomes and Lamp1 positive lysosomes in APAP-treated primary hepatocytes. Moreover, isolated autophagosomes and autolysosomes from APAP-treated mouse livers contained APAP-AD, suggesting autophagy may selectively remove APAP-AD. APAP-AD were detected in both detergent soluble and insoluble pools in APAP-treated mouse livers and hepatocytes. More importantly, pharmacological inhibition of autophagy by leupeptin or chloroquine increased whereas induction of autophagy by Torin 1 decreased serum APAP-AD levels in APAP-treated mice, which correlated with alanine aminotransferase levels and liver necrosis. Furthermore, SQSTM1/p62, an autophagy receptor protein, was recruited to APAP-AD. Adenovirus-mediated shRNA knockdown of SQSTM1/p62 led to increased APAP-AD and necrosis in primary hepatocytes.
Conclusions: Our data indicate that APAP-AD are removed though selective autophagy. Pharmacological induction of autophagy may be a novel promising approach for treating APAP-induced liver injury.
Lay summary: Acetaminophen overdose can form acetaminophen protein adducts and mitochondria damage in hepatocytes resulting in liver injury. Activation of autophagy-lysosomal degradation pathway can help to remove acetaminophen protein adducts. Pharmacological induction of autophagy may be a novel promising approach for treating APAP-induced liver injury.
Keywords: Acetaminophen; Acetaminophen protein adducts; Autophagy; Liver injury; p62/SQSTM1.
Copyright © 2016 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures








References
-
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–548. vi. - PubMed
-
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. - PubMed
-
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

