Adherens Junction and E-Cadherin complex regulation by epithelial polarity
- PMID: 27151512
- PMCID: PMC11108514
- DOI: 10.1007/s00018-016-2260-8
Adherens Junction and E-Cadherin complex regulation by epithelial polarity
Abstract
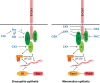
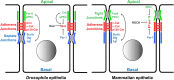
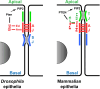
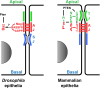
E-Cadherin-based Adherens Junctions (AJs) are a defining feature of all epithelial sheets. Through the homophilic association of E-Cadherin molecules expressed on neighboring cells, they ensure intercellular adhesion amongst epithelial cells, and regulate many key aspects of epithelial biology. While their adhesive role requires these structures to remain stable, AJs are also extremely plastic. This plasticity allows for the adaptation of the cell to its changing environment: changes in neighbors after cell division, cell death, or cell movement, and changes in cell shape during differentiation. In this review we focus on the recent advances highlighting the critical role of the apico-basal polarity machinery, and in particular of the Par3/Bazooka scaffold, in the regulation and remodeling of AJs. We propose that by regulating key phosphorylation events on the core E-Cadherin complex components, Par3 and epithelial polarity promote meta-stable protein complexes governing the correct formation, localization, and functioning of AJ.
Keywords: Adherens Junctions; E-Cadherin; Epithelial polarity; Magi scaffolds; Par3; Remodeling.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

