Personalized Stem Cell Therapy to Correct Corneal Defects Due to a Unique Homozygous-Heterozygous Mosaicism of Ectrodactyly-Ectodermal Dysplasia-Clefting Syndrome
- PMID: 27151912
- PMCID: PMC4954457
- DOI: 10.5966/sctm.2015-0358
Personalized Stem Cell Therapy to Correct Corneal Defects Due to a Unique Homozygous-Heterozygous Mosaicism of Ectrodactyly-Ectodermal Dysplasia-Clefting Syndrome
Abstract
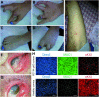
: Ectrodactyly-ectodermal dysplasia-clefting (EEC) syndrome is a rare autosomal dominant disease caused by mutations in the p63 gene. To date, approximately 40 different p63 mutations have been identified, all heterozygous. No definitive treatments are available to counteract and resolve the progressive corneal degeneration due to a premature aging of limbal epithelial stem cells. Here, we describe a unique case of a young female patient, aged 18 years, with EEC and corneal dysfunction, who was, surprisingly, homozygous for a novel and de novo R311K missense mutation in the p63 gene. A detailed analysis of the degree of somatic mosaicism in leukocytes from peripheral blood and oral mucosal epithelial stem cells (OMESCs) from biopsies of buccal mucosa showed that approximately 80% were homozygous mutant cells and 20% were heterozygous. Cytogenetic and molecular analyses excluded genomic alterations, thus suggesting a de novo mutation followed by an allelic gene conversion of the wild-type allele by de novo mutant allele as a possible mechanism to explain the homozygous condition. R311K-p63 OMESCs were expanded in vitro and heterozygous holoclones selected following clonal analysis. These R311K-p63 OMESCs were able to generate well-organized and stratified epithelia in vitro, resembling the features of healthy tissues. This study supports the rationale for the development of cultured autologous oral mucosal epithelial stem cell sheets obtained by selected heterozygous R311K-p63 stem cells, as an effective and personalized therapy for reconstructing the ocular surface of this unique case of EEC syndrome, thus bypassing gene therapy approaches.
Significance: This case demonstrates that in a somatic mosaicism context, a novel homozygous mutation in the p63 gene can arise as a consequence of an allelic gene conversion event, subsequent to a de novo mutation. The heterozygous mutant R311K-p63 stem cells can be isolated by means of clonal analysis and given their good regenerative capacity, they may be used to successfully correct the corneal defects present in this unique case of ectrodactyly-ectodermal dysplasia-clefting syndrome.
Keywords: Cell therapy; Ectrodactyly-ectodermal dysplasia-clefting syndrome; Gene conversion; Mosaicism; p63.
©AlphaMed Press.
Figures



References
-
- Clements SE, Techanukul T, Coman D, et al. Molecular basis of EEC (ectrodactyly, ectodermal dysplasia, clefting) syndrome: Five new mutations in the DNA-binding domain of the TP63 gene and genotype-phenotype correlation. Br J Dermatol. 2010;162:201–207. - PubMed
-
- Di Iorio E, Kaye SB, Ponzin D, et al. Limbal stem cell deficiency and ocular phenotype in ectrodactyly-ectodermal dysplasia-clefting syndrome caused by p63 mutations. Ophthalmology. 2012;119:74–83. - PubMed
-
- Hamada T, Chan I, Willoughby CE, et al. Common mutations in Arg304 of the p63 gene in ectrodactyly, ectodermal dysplasia, clefting syndrome: Lack of genotype-phenotype correlation and implications for mutation detection strategies. J Invest Dermatol. 2002;119:1202–1203. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

