Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD-Related Systemic Inflammation
- PMID: 27151924
- PMCID: PMC5198279
- DOI: 10.1681/ASN.2015111285
Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD-Related Systemic Inflammation
Abstract
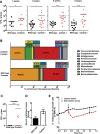
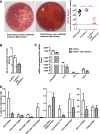
CKD associates with systemic inflammation, but the underlying cause is unknown. Here, we investigated the involvement of intestinal microbiota. We report that collagen type 4 α3-deficient mice with Alport syndrome-related progressive CKD displayed systemic inflammation, including increased plasma levels of pentraxin-2 and activated antigen-presenting cells, CD4 and CD8 T cells, and Th17- or IFNγ-producing T cells in the spleen as well as regulatory T cell suppression. CKD-related systemic inflammation in these mice associated with intestinal dysbiosis of proteobacterial blooms, translocation of living bacteria across the intestinal barrier into the liver, and increased serum levels of bacterial endotoxin. Uremia did not affect secretory IgA release into the ileum lumen or mucosal leukocyte subsets. To test for causation between dysbiosis and systemic inflammation in CKD, we eradicated facultative anaerobic microbiota with antibiotics. This eradication prevented bacterial translocation, significantly reduced serum endotoxin levels, and fully reversed all markers of systemic inflammation to the level of nonuremic controls. Therefore, we conclude that uremia associates with intestinal dysbiosis, intestinal barrier dysfunction, and bacterial translocation, which trigger the state of persistent systemic inflammation in CKD. Uremic dysbiosis and intestinal barrier dysfunction may be novel therapeutic targets for intervention to suppress CKD-related systemic inflammation and its consequences.
Keywords: chronic kidney disease; inflammation; microbiota.
Copyright © 2016 by the American Society of Nephrology.
Figures

Comment in
-
Chronic kidney disease: Microbiota trigger inflammation.Nat Rev Nephrol. 2016 Jul;12(7):376. doi: 10.1038/nrneph.2016.73. Epub 2016 May 23. Nat Rev Nephrol. 2016. PMID: 27211871 No abstract available.
References
-
- Carrero JJ, Stenvinkel P: Inflammation in end-stage renal disease--what have we learned in 10 years? Semin Dial 23: 498–509, 2010 - PubMed
-
- McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S, Owen PJ, Lai KB, Li PK: Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6: 133–141, 2011 - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

