Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy
- PMID: 27151947
- PMCID: PMC4920167
- DOI: 10.1242/dev.129908
Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy
Abstract
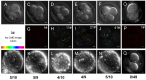
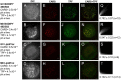
Mammalian oocytes contain lipid droplets that are a store of fatty acids, whose metabolism plays a substantial role in pre-implantation development. Fluorescent staining has previously been used to image lipid droplets in mammalian oocytes and embryos, but this method is not quantitative and often incompatible with live cell imaging and subsequent development. Here we have applied chemically specific, label-free coherent anti-Stokes Raman scattering (CARS) microscopy to mouse oocytes and pre-implantation embryos. We show that CARS imaging can quantify the size, number and spatial distribution of lipid droplets in living mouse oocytes and embryos up to the blastocyst stage. Notably, it can be used in a way that does not compromise oocyte maturation or embryo development. We have also correlated CARS with two-photon fluorescence microscopy simultaneously acquired using fluorescent lipid probes on fixed samples, and found only a partial degree of correlation, depending on the lipid probe, clearly exemplifying the limitation of lipid labelling. In addition, we show that differences in the chemical composition of lipid droplets in living oocytes matured in media supplemented with different saturated and unsaturated fatty acids can be detected using CARS hyperspectral imaging. These results demonstrate that CARS microscopy provides a novel non-invasive method of quantifying lipid content, type and spatial distribution with sub-micron resolution in living mammalian oocytes and embryos.
Keywords: Egg; Embryo; Lipid; Microscopy; Oocyte.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

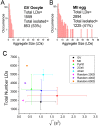
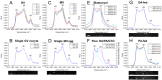
 ) against the total number of LDs, in ensembles of GV oocytes (n=33), MII eggs (n=30), MII eggs starved of pyruvate (PyrSt; n=8), two-cell (2Cell; n=10) and four-cell (4Cell; n=8) embryos (including those represented in Fig. 1). The distribution of each variable in the corresponding ensemble is shown as a mean (symbol) and standard deviation (bar). The case of a random LD distribution simulated for a range of total number of LDs is also shown for comparison.
) against the total number of LDs, in ensembles of GV oocytes (n=33), MII eggs (n=30), MII eggs starved of pyruvate (PyrSt; n=8), two-cell (2Cell; n=10) and four-cell (4Cell; n=8) embryos (including those represented in Fig. 1). The distribution of each variable in the corresponding ensemble is shown as a mean (symbol) and standard deviation (bar). The case of a random LD distribution simulated for a range of total number of LDs is also shown for comparison.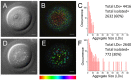
References
-
- Aardema H., Vos P. L. A. M., Lolicato F., Roelen B. A. J., Knijn H. M., Vaandrager A. B., Helms J. B. and Gadella B. M. (2011). Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol. Reprod. 85, 62-69. 10.1095/biolreprod.110.088815 - DOI - PubMed
-
- Del Collado M., Saraiva N. Z., Lopes F. L., Gaspar R. C., Padilha L. C., Costa R. R., Rossi G. F., Vantini R. and Garcia J. M. (2015). Influence of bovine serum albumin and fetal bovine serum supplementation during in vitro maturation on lipid and mitochondrial behaviour in oocytes and lipid accumulation in bovine embryos. Reprod. Fertil. Dev. (in press), 10.1071/rd15067. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

