Dual and Opposing Roles of Xanthine Dehydrogenase in Defense-Associated Reactive Oxygen Species Metabolism in Arabidopsis
- PMID: 27152019
- PMCID: PMC4904670
- DOI: 10.1105/tpc.15.00880
Dual and Opposing Roles of Xanthine Dehydrogenase in Defense-Associated Reactive Oxygen Species Metabolism in Arabidopsis
Abstract
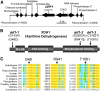
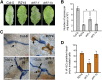
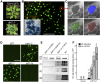
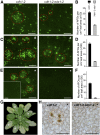
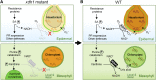
While plants produce reactive oxygen species (ROS) for stress signaling and pathogen defense, they need to remove excessive ROS induced during stress responses in order to minimize oxidative damage. How can plants fine-tune this balance and meet such conflicting needs? Here, we show that XANTHINE DEHYDROGENASE1 (XDH1) in Arabidopsis thaliana appears to play spatially opposite roles to serve this purpose. Through a large-scale genetic screen, we identified three missense mutations in XDH1 that impair XDH1's enzymatic functions and consequently affect the powdery mildew resistance mediated by RESISTANCE TO POWDERY MILDEW8 (RPW8) in epidermal cells and formation of xanthine-enriched autofluorescent objects in mesophyll cells. Further analyses revealed that in leaf epidermal cells, XDH1 likely functions as an oxidase, along with the NADPH oxidases RbohD and RbohF, to generate superoxide, which is dismutated into H2O2 The resulting enrichment of H2O2 in the fungal haustorial complex within infected epidermal cells helps to constrain the haustorium, thereby contributing to RPW8-dependent and RPW8-independent powdery mildew resistance. By contrast, in leaf mesophyll cells, XDH1 carries out xanthine dehydrogenase activity to produce uric acid in local and systemic tissues to scavenge H2O2 from stressed chloroplasts, thereby protecting plants from stress-induced oxidative damage. Thus, XDH1 plays spatially specified dual and opposing roles in modulation of ROS metabolism during defense responses in Arabidopsis.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures
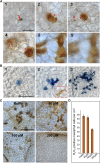
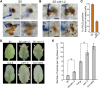
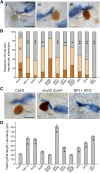
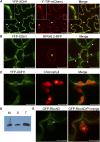
Comment in
-
Opposing Functions for Plant Xanthine Dehydrogenase in Response to Powdery Mildew Infection: Production and Scavenging of Reactive Oxygen Species.Plant Cell. 2016 May;28(5):1001. doi: 10.1105/tpc.16.00381. Epub 2016 May 10. Plant Cell. 2016. PMID: 27166140 Free PMC article. No abstract available.
Similar articles
-
Ectopic expression of RESISTANCE TO POWDERY MILDEW8.1 confers resistance to fungal and oomycete pathogens in Arabidopsis.Plant Cell Physiol. 2014 Aug;55(8):1484-96. doi: 10.1093/pcp/pcu080. Epub 2014 Jun 4. Plant Cell Physiol. 2014. PMID: 24899552
-
Dominant negative RPW8.2 fusion proteins reveal the importance of haustorium-oriented protein trafficking for resistance against powdery mildew in Arabidopsis.Plant Signal Behav. 2015;10(3):e989766. doi: 10.4161/15592324.2014.989766. Plant Signal Behav. 2015. PMID: 25830634 Free PMC article.
-
The powdery mildew resistance protein RPW8.2 is carried on VAMP721/722 vesicles to the extrahaustorial membrane of haustorial complexes.Plant J. 2014 Sep;79(5):835-47. doi: 10.1111/tpj.12591. Epub 2014 Jul 23. Plant J. 2014. PMID: 24941879
-
Regulation of the NADPH Oxidase RBOHD During Plant Immunity.Plant Cell Physiol. 2015 Aug;56(8):1472-80. doi: 10.1093/pcp/pcv063. Epub 2015 May 4. Plant Cell Physiol. 2015. PMID: 25941234 Review.
-
Focal accumulation of defences at sites of fungal pathogen attack.J Exp Bot. 2008;59(13):3501-8. doi: 10.1093/jxb/ern205. Epub 2008 Aug 13. J Exp Bot. 2008. PMID: 18703493 Free PMC article. Review.
Cited by
-
Enzymes and cellular interplay required for flux of fixed nitrogen to ureides in bean nodules.Nat Commun. 2022 Sep 10;13(1):5331. doi: 10.1038/s41467-022-33005-5. Nat Commun. 2022. PMID: 36088455 Free PMC article.
-
Transcription Factor PecS Mediates Agrobacterium fabrum Fitness and Survival.J Bacteriol. 2023 Jul 25;205(7):e0047822. doi: 10.1128/jb.00478-22. Epub 2023 Jun 14. J Bacteriol. 2023. PMID: 37314346 Free PMC article.
-
Opposing Functions for Plant Xanthine Dehydrogenase in Response to Powdery Mildew Infection: Production and Scavenging of Reactive Oxygen Species.Plant Cell. 2016 May;28(5):1001. doi: 10.1105/tpc.16.00381. Epub 2016 May 10. Plant Cell. 2016. PMID: 27166140 Free PMC article. No abstract available.
-
Recent Progress in Understanding the Role of Reactive Oxygen Species in Plant Cell Signaling.Plant Physiol. 2016 Jul;171(3):1535-9. doi: 10.1104/pp.16.00938. Plant Physiol. 2016. PMID: 27385820 Free PMC article. No abstract available.
-
The different tolerance to magnesium deficiency of two grapevine rootstocks relies on the ability to cope with oxidative stress.BMC Plant Biol. 2019 Apr 16;19(1):148. doi: 10.1186/s12870-019-1726-x. BMC Plant Biol. 2019. PMID: 30991946 Free PMC article.
References
-
- Alvarez M.E., Pennell R.I., Meijer P.J., Ishikawa A., Dixon R.A., Lamb C. (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92: 773–784. - PubMed
-
- Bartsch M., Gobbato E., Bednarek P., Debey S., Schultze J.L., Bautor J., Parker J.E. (2006). Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18: 1038–1051. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

