C-O bond Formation in a Microfluidic Reactor: High Yield SNAr Substitution of Heteroaryl Chlorides
- PMID: 27152054
- PMCID: PMC4852388
- DOI: 10.1016/j.tetlet.2016.03.095
C-O bond Formation in a Microfluidic Reactor: High Yield SNAr Substitution of Heteroaryl Chlorides
Abstract
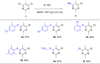
This study describes our development of a novel and efficient procedure for C-O bond formation under mild conditions, for coupling heteroaryl chlorides with phenols or primary aliphatic alcohols. We utilized a continuous-flow microfluidic reactor for C-O bond formation in electron-deficient pyrimidines and pyridines in a much more facile manner with a cleaner reaction profile, high yield, quick scalability and without the need for the transition metal catalyst. This approach can be of general utility to make C-O bond containing intermediates of industrial importance in a continuous and safe manner.
Keywords: C–O bond; Flow chemistry; Micro-fluidic reactor; SNAr reaction.
Figures



Similar articles
-
Facile C-F Bond Formation through a Concerted Nucleophilic Aromatic Substitution Mediated by the PhenoFluor Reagent.Acc Chem Res. 2017 Nov 21;50(11):2822-2833. doi: 10.1021/acs.accounts.7b00413. Epub 2017 Nov 9. Acc Chem Res. 2017. PMID: 29120599
-
Recent Advances in First-Row Transition Metal-Catalyzed Reductive Coupling Reactions for π-Bond Functionalization and C-Glycosylation.Acc Chem Res. 2023 Nov 21;56(22):3292-3312. doi: 10.1021/acs.accounts.3c00531. Epub 2023 Nov 2. Acc Chem Res. 2023. PMID: 37917928
-
Development of SNAr Nucleophilic Fluorination: A Fruitful Academia-Industry Collaboration.Acc Chem Res. 2020 Oct 20;53(10):2372-2383. doi: 10.1021/acs.accounts.0c00471. Epub 2020 Sep 24. Acc Chem Res. 2020. PMID: 32969213
-
Recent Advances in Carbon-Nitrogen/Carbon-Oxygen Bond Formation Under Transition-Metal-Free Conditions.Chem Rec. 2023 May;23(5):e202300020. doi: 10.1002/tcr.202300020. Epub 2023 Mar 30. Chem Rec. 2023. PMID: 36995073 Review.
-
Recent advances in C/N-alkylation with alcohols through hydride transfer strategies.Org Biomol Chem. 2022 Oct 12;20(39):7713-7745. doi: 10.1039/d2ob00706a. Org Biomol Chem. 2022. PMID: 36169049 Review.
Cited by
-
Synthesis of Fused Indolines by Interrupted Fischer Indolization in a Microfluidic Reactor.Tetrahedron Lett. 2019 Jan 17;60(3):322-326. doi: 10.1016/j.tetlet.2018.12.045. Epub 2018 Dec 19. Tetrahedron Lett. 2019. PMID: 30631216 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
