Melatonin Preserves Blood-Brain Barrier Integrity and Permeability via Matrix Metalloproteinase-9 Inhibition
- PMID: 27152411
- PMCID: PMC4859489
- DOI: 10.1371/journal.pone.0154427
Melatonin Preserves Blood-Brain Barrier Integrity and Permeability via Matrix Metalloproteinase-9 Inhibition
Abstract
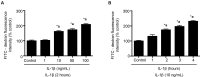
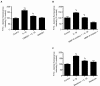
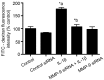
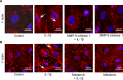
Microvascular hyperpermeability that occurs at the level of the blood-brain barrier (BBB) often leads to vasogenic brain edema and elevated intracranial pressure following traumatic brain injury (TBI). At a cellular level, tight junction proteins (TJPs) between neighboring endothelial cells maintain the integrity of the BBB via TJ associated proteins particularly, zonula occludens-1 (ZO-1) that binds to the transmembrane TJPs and actin cytoskeleton intracellularly. The pro-inflammatory cytokine, interleukin-1β (IL-1β) as well as the proteolytic enzymes, matrix metalloproteinase-9 (MMP-9) are key mediators of trauma-associated brain edema. Recent studies indicate that melatonin a pineal hormone directly binds to MMP-9 and also might act as its endogenous inhibitor. We hypothesized that melatonin treatment will provide protection against TBI-induced BBB hyperpermeability via MMP-9 inhibition. Rat brain microvascular endothelial cells grown as monolayers were used as an in vitro model of the BBB and a mouse model of TBI using a controlled cortical impactor was used for all in vivo studies. IL-1β (10 ng/mL; 2 hours)-induced endothelial monolayer hyperpermeability was significantly attenuated by melatonin (10 μg/mL; 1 hour), GM6001 (broad spectrum MMP inhibitor; 10 μM; 1 hour), MMP-9 inhibitor-1 (MMP-9 specific inhibitor; 5 nM; 1 hour) or MMP-9 siRNA transfection (48 hours) in vitro. Melatonin and MMP-9 inhibitor-1 pretreatment attenuated IL-1β-induced MMP-9 activity, loss of ZO-1 junctional integrity and f-actin stress fiber formation. IL-1β treatment neither affected ZO-1 protein or mRNA expression or cell viability. Acute melatonin treatment attenuated BBB hyperpermeability in a mouse controlled cortical impact model of TBI in vivo. In conclusion, one of the protective effects of melatonin against BBB hyperpermeability occurs due to enhanced BBB integrity via MMP-9 inhibition. In addition, acute melatonin treatment provides protection against BBB hyperpermeability in a mouse model of TBI indicating its potential as a therapeutic agent for brain edema when established in humans.
Conflict of interest statement
Figures





References
-
- Bolton SJ, Anthony DC, Perry VH (1998) Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience 86: 1245–1257. - PubMed
-
- Simi A, Tsakiri N, Wang P, Rothwell NJ (2007) Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans 35: 1122–1126. - PubMed
-
- Didier N, Romero IA, Créminon C, Wijkhuisen A, Grassi J, Mabondzo A. (2004) Secretion of interleukin-1β by astrocytes mediates endothelin-1 and tumour necrosis factor-α effects on human brain microvascular endothelial cell permeability. Journal of Neurochemistry 86: 246–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

