The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer
- PMID: 27152637
- PMCID: PMC4956510
- DOI: 10.1002/cncr.29992
The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer
Abstract
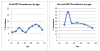
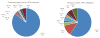
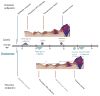
The incidence of oropharyngeal cancer (OPC) is significantly increasing in the United States. Given that these epidemiologic trends are driven by human papillomavirus (HPV), the potential impact of prophylactic HPV vaccines on the prevention of OPC is of interest. The primary evidence supporting the approval of current prophylactic HPV vaccines is from large phase 3 clinical trials focused on the prevention of genital disease (cervical and anal cancer, as well as genital warts). These trials reported vaccine efficacy rates of 89% to 98% for the prevention of both premalignant lesions and persistent genital infections. However, these trials were designed before the etiologic relationship between HPV and OPC was established. There are differences in the epidemiology of oral and genital HPV infection, such as differences in age and sex distributions, which suggest that the vaccine efficacy observed in genital cancers may not be directly translatable to the cancers of the oropharynx. Evaluation of vaccine efficacy is challenging in the oropharynx because no premalignant lesion analogous to cervical intraepithelial neoplasia in cervical cancer has yet been identified. To truly investigate the efficacy of these vaccines in the oropharynx, additional clinical trials with feasible endpoints are needed. Cancer 2016;122:2313-2323. © 2016 American Cancer Society.
Keywords: cancer vaccines; human papillomavirus (HPV); human papillomavirus vaccines; oropharyngeal neoplasms; squamous cell carcinoma of the head and neck.
© 2016 American Cancer Society.
Figures
References
-
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Feb 1;26(4):612–619. - PubMed
-
- Young D, Xiao CC, Murphy B, Moore M, Fakhry C, Day TA. Increase in head and neck cancer in younger patients due to human papillomavirus (HPV) Oral Oncol. 2015 Aug;51(8):727–730. - PubMed
-
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013 Mar;40(3):187–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

