Intravenous Ibuprofen for Treatment of Post-Operative Pain: A Multicenter, Double Blind, Placebo-Controlled, Randomized Clinical Trial
- PMID: 27152748
- PMCID: PMC4859493
- DOI: 10.1371/journal.pone.0154004
Intravenous Ibuprofen for Treatment of Post-Operative Pain: A Multicenter, Double Blind, Placebo-Controlled, Randomized Clinical Trial
Abstract
Background: Non-steroidal anti-inflammatory drugs are often used as components of multimodal therapy for postoperative pain management, but their use is currently limited by its side effects. The specific objective of this study was to evaluate the efficacy and safety of a new formulation of intravenous (IV) ibuprofen for the management of postoperative pain in a European population.
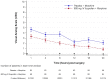
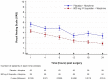
Methods and findings: A total of 206 patients from both abdominal and orthopedic surgery, were randomly assigned in 1:1 ratio to receive 800 mg IV-ibuprofen or placebo every 6 hours; all patients had morphine access through a patient controlled analgesia pump. The primary outcome measure was median morphine consumption within the first 24 hours following surgery. The mean±SEM of morphine requirements was reduced from 29,8±5,25 mg to 14,22±3,23 mg (p = 0,015) and resulted in a decrease in pain at rest (p = 0,02) measured by Visual Analog Scale (VAS) from mean±SEM 3.34±0,35 to 0.86±0.24, and also in pain during movement (p = 0,02) from 4.32±0,36 to 1.90±0,30 in the ibuprofen treatment arm; while in the placebo group VAS score at rest ranged from 4.68±0,40 to 2.12±0,42 and during movement from 5.66±0,42 to 3.38±0,44. Similar treatment-emergent adverse events occurred across both study groups and there was no difference in the overall incidence of these events.
Conclusions: Perioperative administration of IV-Ibuprofen 800 mg every 6 hours in abdominal surgery patient's decreases morphine requirements and pain score. Furthermore IV-Ibuprofen was safe and well tolerate. Consequently we consider appropriate that protocols for management of postoperative pain include IV-Ibuprofen 800 mg every 6 hours as an option to offer patients an analgesic benefit while reducing the potentially risks associated with morphine consumption.
Trial registration: EU Clinical Trials Register 2011-005007-33.
Conflict of interest statement
Figures

References
-
- Rosa-Díaz J, Navarrete-Zuazo V, Díaz-Mendiondo M. Aspectos básicos del dolor postoperatorio y la analgesia multimodal preventiva. Revista Mexicana de Anestesiología. 2014;37: 18–26.
-
- Ducharme J. Acute pain and pain control: State of the art. Ann Emerg Med. 2000;35: 592–603. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

