Autophagy, Innate Immunity and Tissue Repair in Acute Kidney Injury
- PMID: 27153058
- PMCID: PMC4881488
- DOI: 10.3390/ijms17050662
Autophagy, Innate Immunity and Tissue Repair in Acute Kidney Injury
Abstract
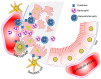
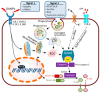
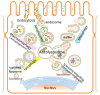
Kidney is a vital organ with high energy demands to actively maintain plasma hemodynamics, electrolytes and water homeostasis. Among the nephron segments, the renal tubular epithelium is endowed with high mitochondria density for their function in active transport. Acute kidney injury (AKI) is an important clinical syndrome and a global public health issue with high mortality rate and socioeconomic burden due to lack of effective therapy. AKI results in acute cell death and necrosis of renal tubule epithelial cells accompanied with leakage of tubular fluid and inflammation. The inflammatory immune response triggered by the tubular cell death, mitochondrial damage, associative oxidative stress, and the release of many tissue damage factors have been identified as key elements driving the pathophysiology of AKI. Autophagy, the cellular mechanism that removes damaged organelles via lysosome-mediated degradation, had been proposed to be renoprotective. An in-depth understanding of the intricate interplay between autophagy and innate immune response, and their roles in AKI pathology could lead to novel therapies in AKI. This review addresses the current pathophysiology of AKI in aspects of mitochondrial dysfunction, innate immunity, and molecular mechanisms of autophagy. Recent advances in renal tissue regeneration and potential therapeutic interventions are also discussed.
Keywords: ischemia-reperfusion injury; kidney disease; nephrotoxicity; oxidative stress; tissue regeneration.
Figures
References
-
- Mehta R.L., Cerda J., Burdmann E.A., Tonelli M., Garcia-Garcia G., Jha V., Susantitaphong P., Rocco M., Vanholder R., Sever M.S., et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

