Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects
- PMID: 27153087
- PMCID: PMC4885049
- DOI: 10.3390/toxins8050134
Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects
Abstract
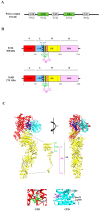
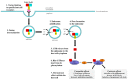
Clostridium difficile infection (CDI) has significant clinical impact especially on the elderly and/or immunocompromised patients. The pathogenicity of Clostridium difficile is mainly mediated by two exotoxins: toxin A (TcdA) and toxin B (TcdB). These toxins primarily disrupt the cytoskeletal structure and the tight junctions of target cells causing cell rounding and ultimately cell death. Detectable C. difficile toxemia is strongly associated with fulminant disease. However, besides the well-known intestinal damage, recent animal and in vitro studies have suggested a more far-reaching role for these toxins activity including cardiac, renal, and neurologic impairment. The creation of C. difficile strains with mutations in the genes encoding toxin A and B indicate that toxin B plays a major role in overall CDI pathogenesis. Novel insights, such as the role of a regulator protein (TcdE) on toxin production and binding interactions between albumin and C. difficile toxins, have recently been discovered and will be described. Our review focuses on the toxin-mediated pathogenic processes of CDI with an emphasis on recent studies.
Keywords: Clostridium difficile; pathogenesis; toxins.
Figures
References
-
- Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

