Zebrafish Sensitivity to Botulinum Neurotoxins
- PMID: 27153088
- PMCID: PMC4885047
- DOI: 10.3390/toxins8050132
Zebrafish Sensitivity to Botulinum Neurotoxins
Abstract
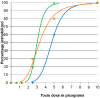
Botulinum neurotoxins (BoNT) are the most potent known toxins. The mouse LD50 assay is the gold standard for testing BoNT potency, but is not sensitive enough to detect the extremely low levels of neurotoxin that may be present in the serum of sensitive animal species that are showing the effects of BoNT toxicity, such as channel catfish affected by visceral toxicosis of catfish. Since zebrafish are an important animal model for diverse biomedical and basic research, they are readily available and have defined genetic lines that facilitate reproducibility. This makes them attractive for use as an alternative bioassay organism. The utility of zebrafish as a bioassay model organism for BoNT was investigated. The 96 h median immobilizing doses of BoNT/A, BoNT/C, BoNT/E, and BoNT/F for adult male Tübingen strain zebrafish (0.32 g mean weight) at 25 °C were 16.31, 124.6, 4.7, and 0.61 picograms (pg)/fish, respectively. These findings support the use of the zebrafish-based bioassays for evaluating the presence of BoNT/A, BoNT/E, and BoNT/F. Evaluating the basis of the relatively high resistance of zebrafish to BoNT/C and the extreme sensitivity to BoNT/F may reveal unique functional patterns to the action of these neurotoxins.
Keywords: botulinum neurotoxins; lethal dose 50; zebrafish.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

