Phage as a Genetically Modifiable Supramacromolecule in Chemistry, Materials and Medicine
- PMID: 27153341
- PMCID: PMC4922537
- DOI: 10.1021/acs.accounts.5b00557
Phage as a Genetically Modifiable Supramacromolecule in Chemistry, Materials and Medicine
Abstract
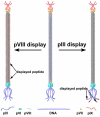
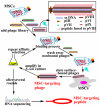
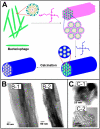
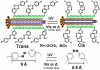
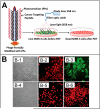
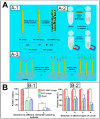
Filamentous bacteriophage (phage) is a genetically modifiable supramacromolecule. It can be pictured as a semiflexible nanofiber (∼900 nm long and ∼8 nm wide) made of a DNA core and a protein shell with the former genetically encoding the latter. Although phage bioengineering and phage display techniques were developed before the 1990s, these techniques have not been widely used for chemistry, materials, and biomedical research from the perspective of supramolecular chemistry until recently. Powered by our expertise in displaying a foreign peptide on its surface through engineering phage DNA, we have employed phage to identify target-specific peptides, construct novel organic-inorganic nanohybrids, develop biomaterials for disease treatment, and generate bioanalytical methods for disease diagnosis. Compared with conventional biomimetic chemistry, phage-based supramolecular chemistry represents a new frontier in chemistry, materials science, and medicine. In this Account, we introduce our recent successful efforts in phage-based supramolecular chemistry, by integrating the unique nanofiber-like phage structure and powerful peptide display techniques into the fields of chemistry, materials science, and medicine: (1) successfully synthesized and assembled silica, hydroxyapatite, and gold nanoparticles using phage templates to form novel functional materials; (2) chemically introduced azo units onto the phage to form photoresponsive functional azo-phage nanofibers via a diazotization reaction between aromatic amino groups and the tyrosine residues genetically displayed on phage surfaces; (3) assembled phage into 2D films for studying the effects of both biochemical (the peptide sequences displayed on the phages) and biophysical (the topographies of the phage films) cues on the proliferation and differentiation of mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs) and identified peptides and topographies that can induce their osteogenic differentiation; (4) discovered that phage could induce angiogenesis and osteogenesis for MSC-based vascularized bone regeneration; (5) identified novel breast cancer cell-targeting and MSC-targeting peptides and used them to significantly improve the efficiency of targeted cancer therapy and MSC-based gene delivery, respectively; (6) employed engineered phage as a probe to achieve ultrasensitive detection of biomarkers from serum of human patients for disease diagnosis; and (7) constructed centimeter-scale 3D multilayered phage assemblies with the potential application as scaffolds for bone regeneration and functional device fabrication. Our findings demonstrated that phage is indeed a very powerful supramacromolecule suitable for not only developing novel nanostructures and biomaterials but also advancing important fields in biomedicine, including molecular targeting, cancer diagnosis and treatment, drug and gene delivery, stem cell fate direction, and tissue regeneration. Our successes in exploiting phage in chemistry, materials, and medicine suggest that phage itself is nontoxic at the cell level and can be safely used for detecting biomarkers in vitro. Moreover, although we have demonstrated successful in vivo tissue regeneration induced by phage, we believe future studies are needed to evaluate the in vivo biodistribution and potential risks of the phage-based biomaterials.
Figures





References
-
- Smith GP, Petrenko VA. Phage Display. Chem. Rev. 1997;97:391–410. - PubMed
-
- Smith GP. Filamentous Fusion Phage - Novel Expression Vectors That Display Cloned Antigens on the Virion Surface. Science. 1985;228:1315–1317. - PubMed
-
- Mao CB, Solis DJ, Reiss BD, Kottmann ST, Sweeney RY, Hayhurst A, Georgiou G, Iverson B, Belcher AM. Virus-based Toolkit for the Directed Synthesis of Magnetic and Semiconducting Nanowires. Science. 2004;303:213–217. - PubMed
-
- Cao BR, Mao CB. Identification of Microtubule-Binding Domains on Microtubule-Associated Proteins by Major Coat Phage Display Technique. Biomacromolecules. 2009;10:555–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

