Inhibition of DHHC20-Mediated EGFR Palmitoylation Creates a Dependence on EGFR Signaling
- PMID: 27153536
- PMCID: PMC4860254
- DOI: 10.1016/j.molcel.2016.04.003
Inhibition of DHHC20-Mediated EGFR Palmitoylation Creates a Dependence on EGFR Signaling
Abstract
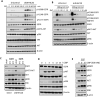
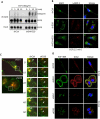
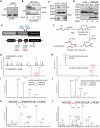
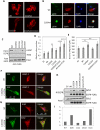
Inappropriate activation of the receptor tyrosine kinase EGFR contributes to a variety of human malignancies. Here we show a mechanism to induce vulnerability to an existing first line treatment for EGFR-driven cancers. We find that inhibiting the palmitoyltransferase DHHC20 creates a dependence on EGFR signaling for cancer cell survival. The loss of palmitoylation increases sustained EGFR signal activation and sensitizes cells to EGFR tyrosine kinase inhibition. Our work shows that the reversible modification of EGFR with palmitate "pins" the unstructured C-terminal tail to the plasma membrane, impeding EGFR activation. We identify by mass spectrometry palmitoylated cysteine residues within the C-terminal tail where mutation of the cysteine residues to alanine is sufficient to activate EGFR signaling promoting cell migration and transformation. Our results reveal that the targeting of a peripheral modulator of EGFR signaling, DHHC20, causes a loss of signal regulation and susceptibility to EGFR inhibitor-induced cell death.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Aicart-Ramos C, Valero RA, Rodriguez-Crespo I. Protein palmitoylation and subcellular trafficking. Biochimica et biophysica acta. 2011;1808:2981–2994. - PubMed
-
- Cho J, Pastorino S, Zeng Q, Xu X, Johnson W, Vandenberg S, Verhaak R, Cherniack AD, Watanabe H, Dutt A, et al. Glioblastoma-derived epidermal growth factor receptor carboxyl-terminal deletion mutants are transforming and are sensitive to EGFR-directed therapies. Cancer research. 2011;71:7587–7596. - PMC - PubMed
-
- Ekstrand AJ, Sugawa N, James CD, Collins VP. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4309–4313. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

