Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells
- PMID: 27153799
- PMCID: PMC4859988
- DOI: 10.1186/s12967-016-0881-1
Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells
Abstract
Background: Adipose-derived stem cells (ASCs) have been identified as a population of multipotent cells with promising applications in tissue engineering and regenerative medicine. ASCs are abundant in fat tissue, which can be safely harvested through the minimally invasive procedure of liposuction. However, there exist a variety of different harvesting methods, with unclear impact on ASC regenerative potential. The aim of this study was thus to compare the functionality of ASCs derived from the common technique of suction-assisted lipoaspiration (SAL) versus resection.
Methods: Human adipose tissue was obtained from paired abdominoplasty and SAL samples from three female donors, and was processed to isolate the stromal vascular fraction. Fluorescence-activated cell sorting was used to determine ASC yield, and cell viability was assayed. Adipogenic and osteogenic differentiation capacity were assessed in vitro using phenotypic staining and quantification of gene expression. Finally, ASCs were applied in an in vivo model of tissue repair to evaluate their regenerative potential.
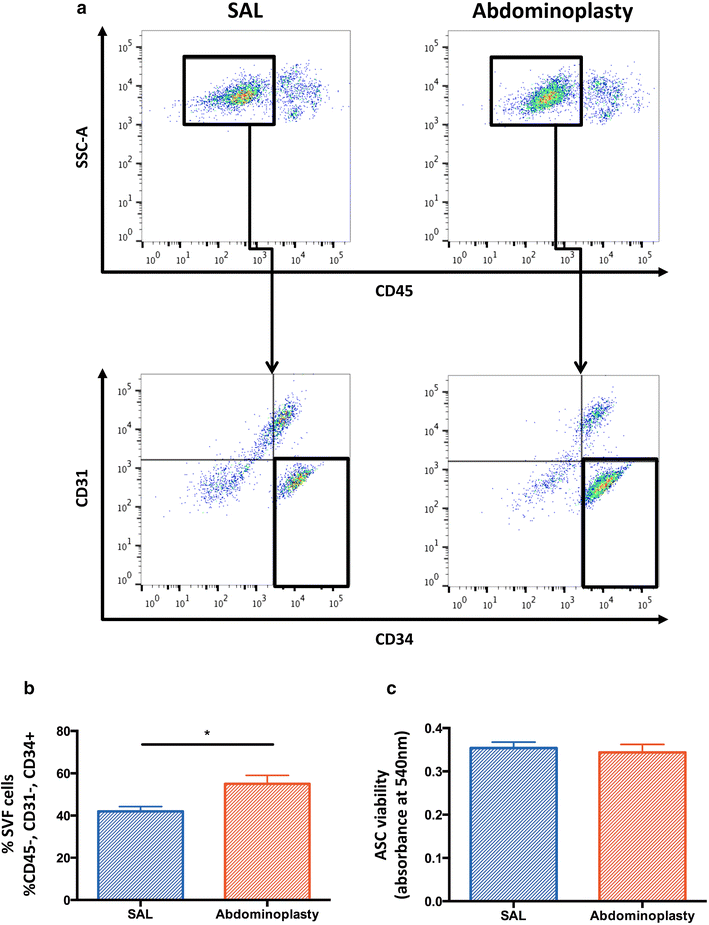
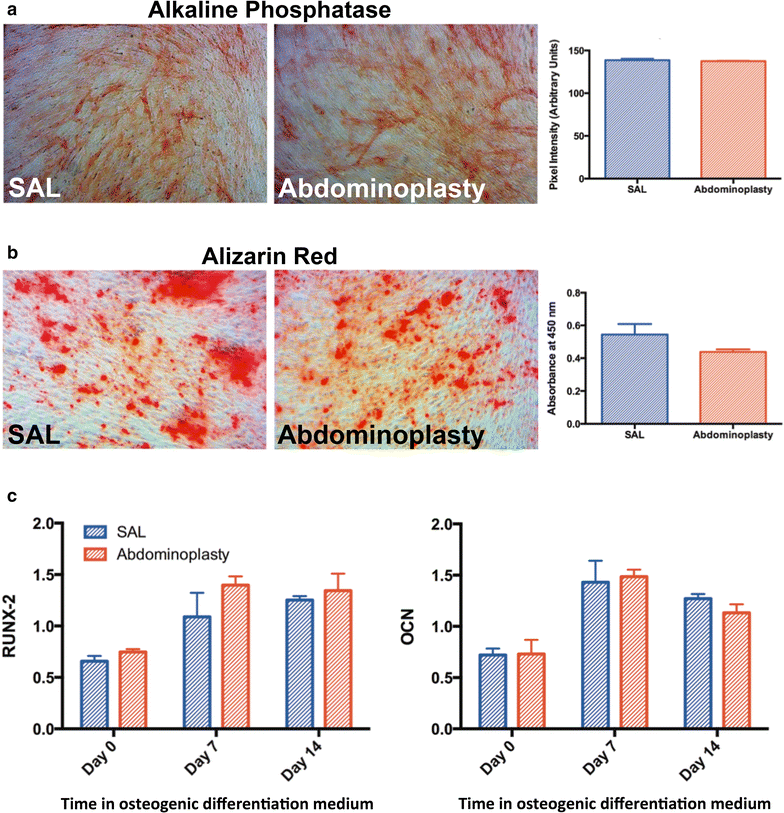
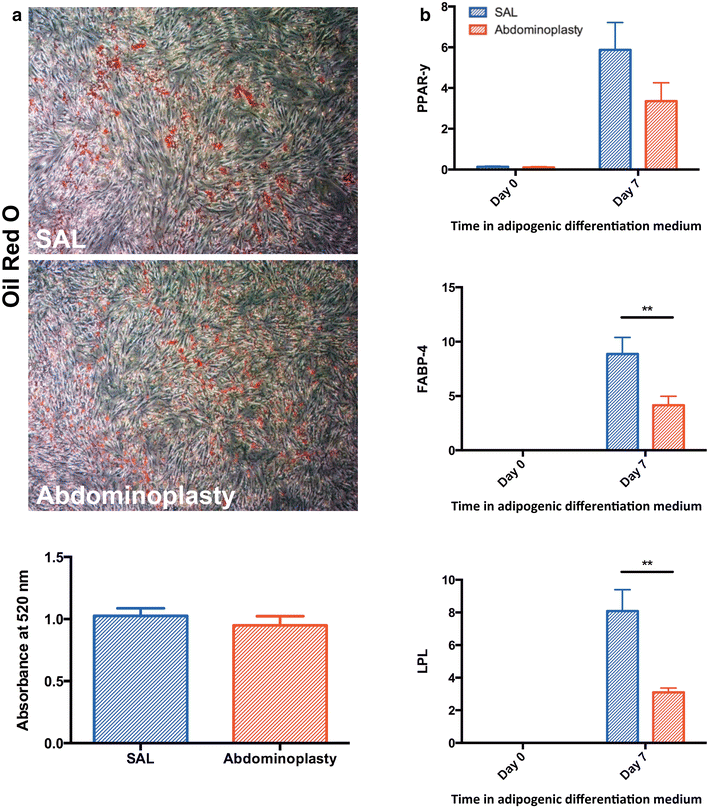
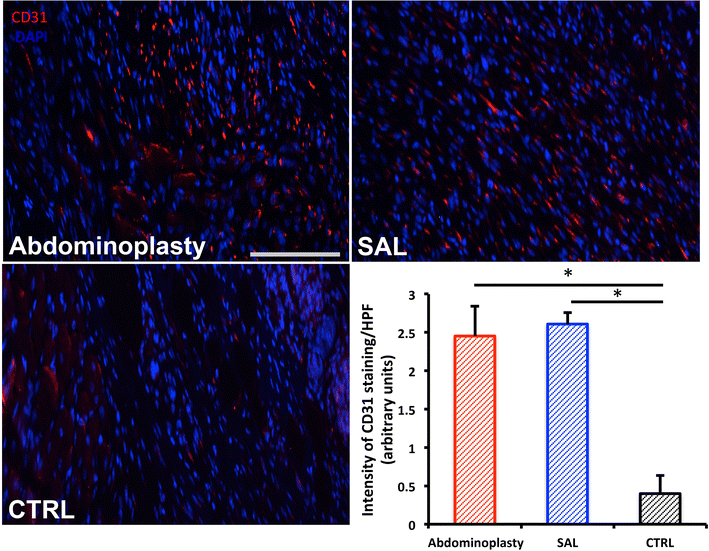
Results: SAL specimens provided significantly fewer ASCs when compared to excised fat tissue, however, with equivalent viability. SAL-derived ASCs demonstrated greater expression of the adipogenic markers FABP-4 and LPL, although this did not result in a difference in adipogenic differentiation. There were no differences detected in osteogenic differentiation capacity as measured by alkaline phosphatase, mineralization or osteogenic gene expression. Both SAL- and resection-derived ASCs enhanced significantly cutaneous healing and vascularization in vivo, with no significant difference between the two groups.
Conclusion: SAL provides viable ASCs with full capacity for multi-lineage differentiation and tissue regeneration, and is an effective method of obtaining ASCs for cell-based therapies.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

