Stat3 Controls Tubulointerstitial Communication during CKD
- PMID: 27153926
- PMCID: PMC5118479
- DOI: 10.1681/ASN.2015091014
Stat3 Controls Tubulointerstitial Communication during CKD
Abstract
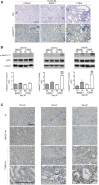
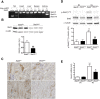
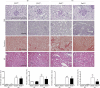
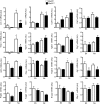
In CKD, tubular cells may be involved in the induction of interstitial fibrosis, which in turn, leads to loss of renal function. However, the molecular mechanisms that link tubular cells to the interstitial compartment are not clear. Activation of the Stat3 transcription factor has been reported in tubular cells after renal damage, and Stat3 has been implicated in CKD progression. Here, we combined an experimental model of nephron reduction in mice from different genetic backgrounds and genetically modified animals with in silico and in vitro experiments to determine whether the selective activation of Stat3 in tubular cells is involved in the development of interstitial fibrosis. Nephron reduction caused Stat3 phosphorylation in tubular cells of lesion-prone mice but not in resistant mice. Furthermore, specific deletion of Stat3 in tubular cells significantly reduced the extent of interstitial fibrosis, which correlated with reduced fibroblast proliferation and matrix synthesis, after nephron reduction. Mechanistically, in vitro tubular Stat3 activation triggered the expression of a specific subset of paracrine profibrotic factors, including Lcn2, Pdgfb, and Timp1. Together, our results provide a molecular link between tubular and interstitial cells during CKD progression and identify Stat3 as a central regulator of this link and a promising therapeutic target.
Keywords: Cell Signaling; Pathophysiology of Renal Disease and Progression; chronic kidney disease; renal fibrosis; renal tubular epithelial cells; transcriptional profiling.
Copyright © 2016 by the American Society of Nephrology.
Figures




Comment in
-
Beyond EMT: Epithelial STAT3 as a Central Regulator of Fibrogenesis.J Am Soc Nephrol. 2016 Dec;27(12):3502-3504. doi: 10.1681/ASN.2016060603. Epub 2016 Jul 1. J Am Soc Nephrol. 2016. PMID: 27371721 Free PMC article. No abstract available.
References
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 - PubMed
-
- Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF: All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 371: 2173–2182, 2008 - PubMed
-
- Risdon RA, Sloper JC, De Wardener HE: Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2: 363–366, 1968 - PubMed
-
- Coppo R, D’Amico G: Factors predicting progression of IgA nephropathies. J Nephrol 18: 503–512, 2005 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

