HIV Exposure to the Epithelia in Ectocervical and Colon Tissues Induces Inflammatory Cytokines Without Tight Junction Disruption
- PMID: 27153934
- PMCID: PMC5067867
- DOI: 10.1089/AID.2015.0185
HIV Exposure to the Epithelia in Ectocervical and Colon Tissues Induces Inflammatory Cytokines Without Tight Junction Disruption
Abstract
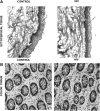
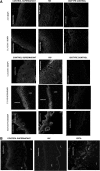
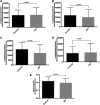
Epithelial cells in human cervical and colonic mucosa do not express HIV receptor. However, HIV transmission occurs across the unbreached epithelia by an unknown mechanism. In this study, the effect of HIV exposure on tight junction (TJ) and cytokine production in ectocervical and colon mucosal epithelia in tissue biopsies was investigated in an organ culture model. After HIV exposure, the distribution patterns and quantities of epithelial TJ and adherens proteins were evaluated by immunofluorescence staining followed by confocal microscopy. Cytokine mRNA in the mucosal epithelia was also evaluated by real-time reverse transcription-polymerase chain reaction (RT-PCR). HIV transmission was evaluated by measuring p24 production in culture supernatant. Our results showed there were no significant changes in the distribution and quantities of epithelial TJ/adherens junction (AJ) proteins after exposure to HIV. However, higher levels of CXCL10 and CXCL11 mRNA expression were detected in HIV-exposed ectocervical epithelia. In case of colon mucosa, higher levels of CXCL10 and IL-6 mRNA expression were detected in HIV-exposed colon mucosa. Our study suggests that HIV induces cytokine production in epithelial cells, which may facilitate HIV transmission by recruiting HIV target cells in the submucosal region. Furthermore, HIV transmission may not occur through epithelial TJ/AJ disruption.
Keywords: HIV transmission; inflammatory cytokines; mucosal epithelia; tight junctions.
Conflict of interest statement
Author Disclosure Statement No competing financial interests exist.
Figures




Similar articles
-
Neisseria gonorrhoeae uses cellular proteins CXCL10 and IL8 to enhance HIV-1 transmission across cervical mucosa.Am J Reprod Immunol. 2019 Jun;81(6):e13111. doi: 10.1111/aji.13111. Epub 2019 Apr 11. Am J Reprod Immunol. 2019. PMID: 30903720 Free PMC article.
-
Structural and functional alteration of corneal epithelial barrier under inflammatory conditions.Curr Eye Res. 2012 Nov;37(11):971-81. doi: 10.3109/02713683.2012.700756. Epub 2012 Jun 27. Curr Eye Res. 2012. PMID: 22738643
-
Human seminal plasma stimulates the migration of CD11c+ mononuclear phagocytes to the apical side of the colonic epithelium without altering the junctional complexes in an ex vivo human intestinal model.Front Immunol. 2023 Mar 22;14:1133886. doi: 10.3389/fimmu.2023.1133886. eCollection 2023. Front Immunol. 2023. PMID: 37033941 Free PMC article.
-
Applications of imaging techniques to studies of epithelial tight junctions.Adv Drug Deliv Rev. 2005 Jan 2;57(1):111-21. doi: 10.1016/j.addr.2004.08.004. Adv Drug Deliv Rev. 2005. PMID: 15518924 Review.
-
The epithelium in inflammatory bowel disease: potential role of endocytosis of junctional proteins in barrier disruption.Novartis Found Symp. 2004;263:115-24; discussion 124-32, 211-8. Novartis Found Symp. 2004. PMID: 15669638 Review.
Cited by
-
Vaccine-Associated Maintenance of Epithelial Integrity Correlated With Protection Against Virus Entry.J Infect Dis. 2018 Sep 8;218(8):1272-1283. doi: 10.1093/infdis/jiy062. J Infect Dis. 2018. PMID: 29401315 Free PMC article.
-
IL-17A reprograms intestinal epithelial cells to facilitate HIV-1 replication and outgrowth in CD4+ T cells.iScience. 2021 Oct 7;24(11):103225. doi: 10.1016/j.isci.2021.103225. eCollection 2021 Nov 19. iScience. 2021. PMID: 34712922 Free PMC article.
-
Inefficient HIV-1 trans Infection of CD4+ T Cells by Macrophages from HIV-1 Nonprogressors Is Associated with Altered Membrane Cholesterol and DC-SIGN.J Virol. 2018 Jun 13;92(13):e00092-18. doi: 10.1128/JVI.00092-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29643243 Free PMC article.
-
Neisseria gonorrhoeae uses cellular proteins CXCL10 and IL8 to enhance HIV-1 transmission across cervical mucosa.Am J Reprod Immunol. 2019 Jun;81(6):e13111. doi: 10.1111/aji.13111. Epub 2019 Apr 11. Am J Reprod Immunol. 2019. PMID: 30903720 Free PMC article.
-
Comparison of Mucosal Markers of Human Immunodeficiency Virus Susceptibility in Healthy Premenopausal Versus Postmenopausal Women.AIDS Res Hum Retroviruses. 2017 Aug;33(8):807-819. doi: 10.1089/AID.2016.0320. Epub 2017 May 16. AIDS Res Hum Retroviruses. 2017. PMID: 28398069 Free PMC article.
References
-
- Kaushic C: HIV-1 infection in the female reproductive tract: Role of interactions between HIV-1 and genital epithelial cells. Am J Reprod Immunol 2011;65:253–260 - PubMed
-
- Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P: Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med 2000;6:475–479 - PubMed
-
- Hladik F, Hope TJ: HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep 2009;6:20–28 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

