Oral treatment with a zinc complex of acetylsalicylic acid prevents diabetic cardiomyopathy in a rat model of type-2 diabetes: activation of the Akt pathway
- PMID: 27153943
- PMCID: PMC4858866
- DOI: 10.1186/s12933-016-0383-8
Oral treatment with a zinc complex of acetylsalicylic acid prevents diabetic cardiomyopathy in a rat model of type-2 diabetes: activation of the Akt pathway
Abstract
Background: Type-2 diabetics have an increased risk of cardiomyopathy, and heart failure is a major cause of death among these patients. Growing evidence indicates that proinflammatory cytokines may induce the development of insulin resistance, and that anti-inflammatory medications may reverse this process. We investigated the effects of the oral administration of zinc and acetylsalicylic acid, in the form of bis(aspirinato)zinc(II)-complex Zn(ASA)2, on different aspects of cardiac damage in Zucker diabetic fatty (ZDF) rats, an experimental model of type-2 diabetic cardiomyopathy.
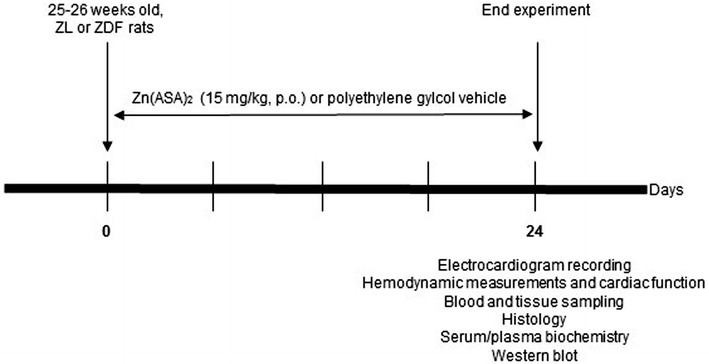
Methods: Nondiabetic control (ZL) and ZDF rats were treated orally with vehicle or Zn(ASA)2 for 24 days. At the age of 29-30 weeks, the electrical activities, left-ventricular functional parameters and left-ventricular wall thicknesses were assessed. Nitrotyrosine immunohistochemistry, TUNEL-assay, and hematoxylin-eosin staining were performed. The protein expression of the insulin-receptor and PI3K/AKT pathway were quantified by Western blot.
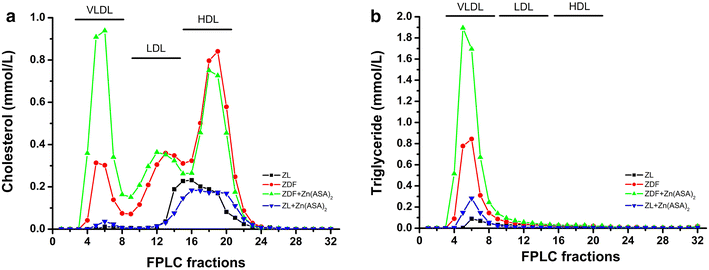
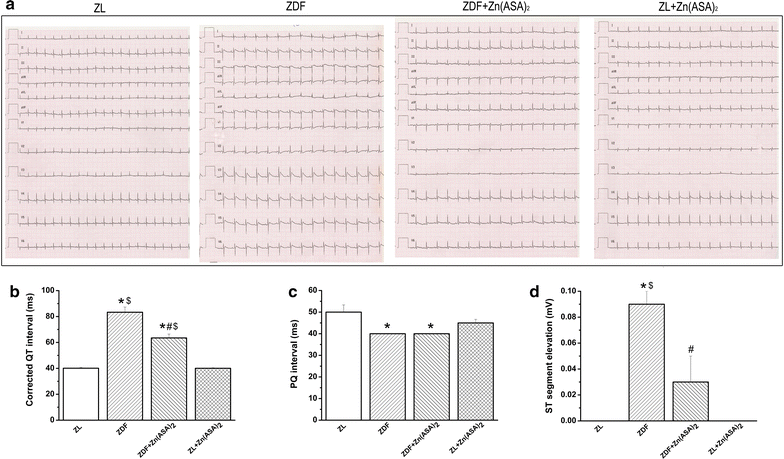
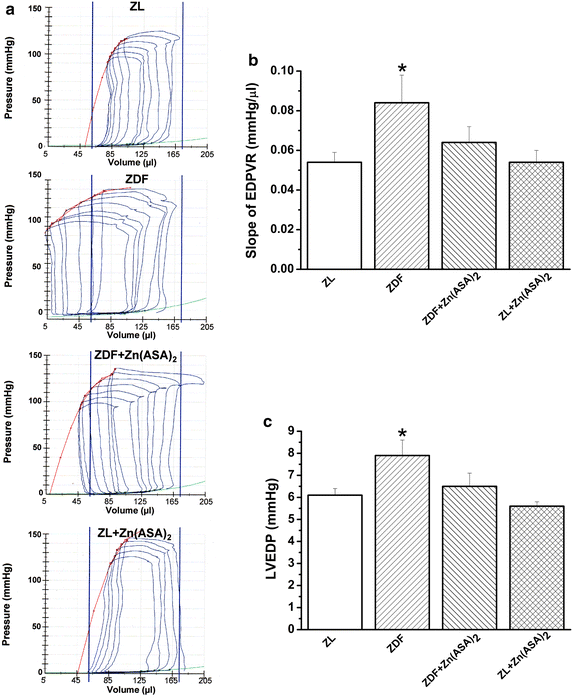
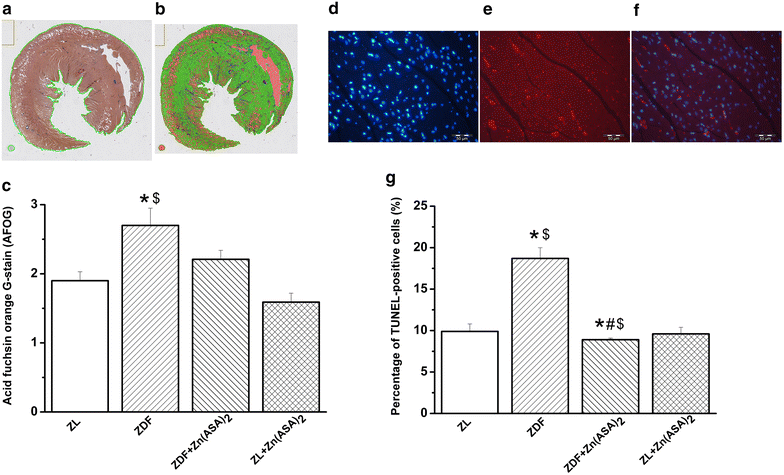
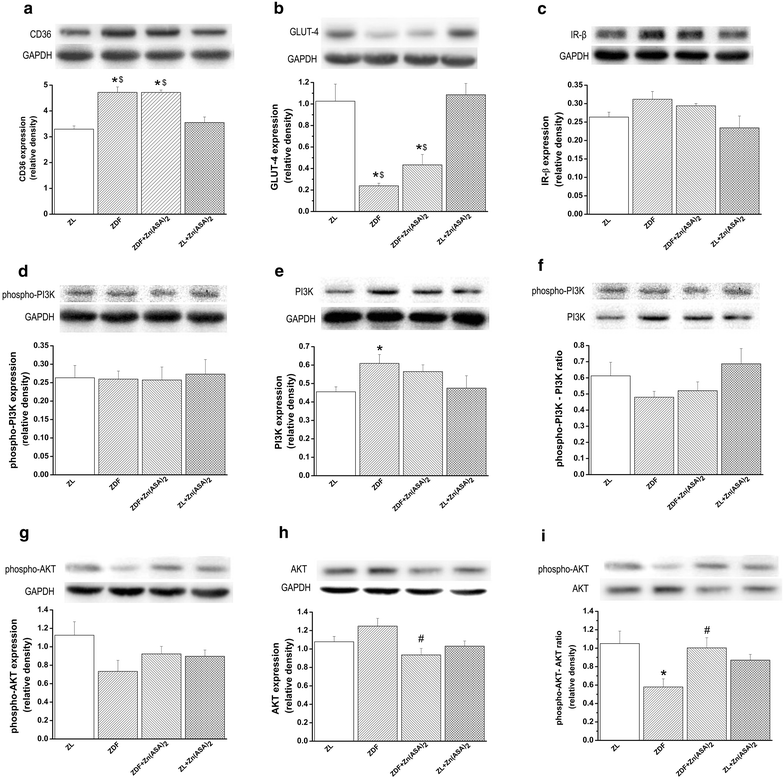
Results: Zn(ASA)2-treatment significantly decreased plasma glucose concentration in ZDF rats (39.0 ± 3.6 vs 49.4 ± 2.8 mM, P < 0.05) while serum insulin-levels were similar among the groups. Data from cardiac catheterization showed that Zn(ASA)2 normalized the increased left-ventricular diastolic stiffness (end-diastolic pressure-volume relationship: 0.064 ± 0.008 vs 0.084 ± 0.014 mmHg/µl; end-diastolic pressure: 6.5 ± 0.6 vs 7.9 ± 0.7 mmHg, P < 0.05). Furthermore, ECG-recordings revealed a restoration of prolonged QT-intervals (63 ± 3 vs 83 ± 4 ms, P < 0.05) with Zn(ASA)2. Left-ventricular wall thickness, assessed by echocardiography, did not differ among the groups. However histological examination revealed an increase in the cardiomyocytes' transverse cross-section area in ZDF compared to the ZL rats, which was significantly decreased after Zn(ASA)2-treatment. Additionally, a significant fibrotic remodeling was observed in the diabetic rats compared to ZL rats, and Zn(ASA)2-administered ZDF rats showed a similar collagen content as ZL animals. In diabetic hearts Zn(ASA)2 significantly decreased DNA-fragmentation, and nitro-oxidative stress, and up-regulated myocardial phosphorylated-AKT/AKT protein expression. Zn(ASA)2 reduced cardiomyocyte death in a cellular model of oxidative stress. Zn(ASA)2 had no effects on altered myocardial CD36, GLUT-4, and PI3K protein expression.
Conclusions: We demonstrated that treatment of type-2 diabetic rats with Zn(ASA)2 reduced plasma glucose-levels and prevented diabetic cardiomyopathy. The increased myocardial AKT activation could, in part, help to explain the cardioprotective effects of Zn(ASA)2. The oral administration of Zn(ASA)2 may have therapeutic potential, aiming to prevent/treat cardiac complications in type-2 diabetic patients.
Keywords: Cardiac function; Diabetic cardiomyopathy; Type-2 diabetes mellitus; Zinc-aspirin complex.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

