Steroids in fluid and/or vasoactive infusion dependent pediatric shock: study protocol for a randomized controlled trial
- PMID: 27153945
- PMCID: PMC4859989
- DOI: 10.1186/s13063-016-1365-6
Steroids in fluid and/or vasoactive infusion dependent pediatric shock: study protocol for a randomized controlled trial
Abstract
Background: Physicians often administer corticosteroids for the treatment of fluid and vasoactive infusion dependent pediatric shock. This use of corticosteroids is controversial, however, and has never been studied in a pediatric randomized controlled trial (RCT). This pilot trial will determine the feasibility of a larger RCT on the role of corticosteroids in pediatric shock.
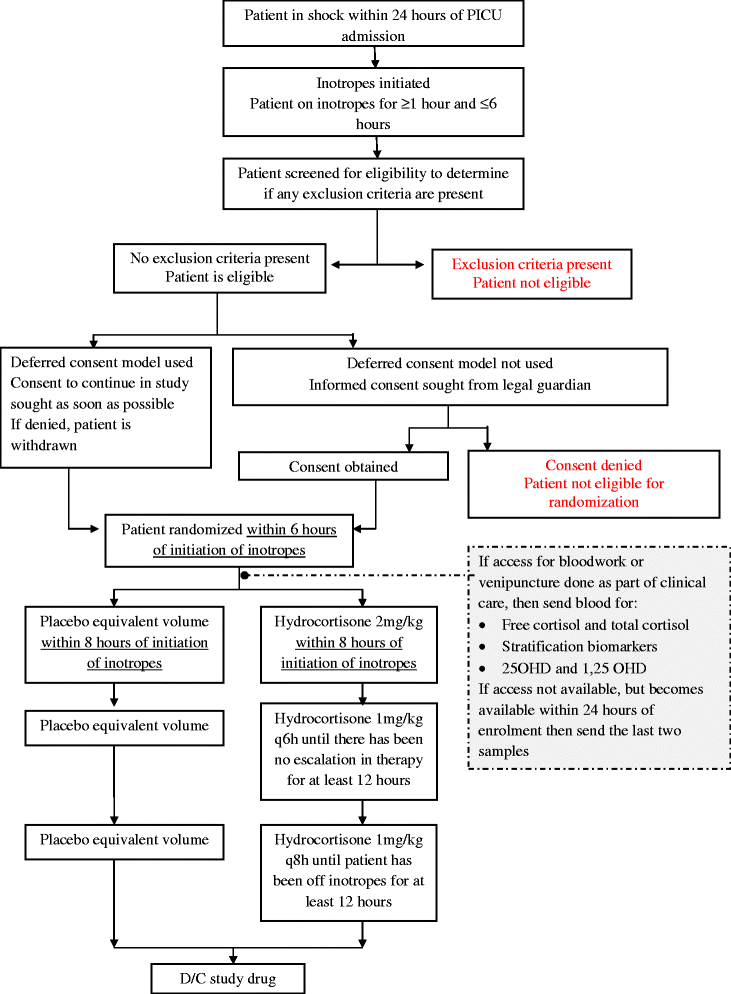
Methods/design: Steroids in Fluid and/or Vasoactive Infusion Dependent Pediatric Shock (STRIPES) is a pragmatic, seven-center, double-blind, pilot RCT. We aim to randomize 72 pediatric patients with fluid and vasoactive infusion dependent shock to receive either hydrocortisone or a saline placebo for 7 days or until clinical stability, whichever occurs first. The primary outcome of this pilot trial is the feasibility of recruitment, defined as the number of patients enrolled over a 1-year period. Secondary outcomes include the frequency of, and reasons for, open-label steroid use, protocol adherence, incidence of mortality and corticosteroid-associated adverse events, time to discontinuation of inotropes, and feasibility of blood sampling.
Discussion: Corticosteroids are used for the treatment of pediatric shock without sufficient evidence to support this practice. While there is a scientific rationale and limited data supporting their use in this setting, there is also evidence from other populations suggesting potential harm. The STRIPES pilot study will assess the feasibility of a larger, much needed trial powered for clinically important outcomes.
Trial registration: ClinicalTrials.gov: NCT02044159.
Figures
Similar articles
-
A trial to determine whether septic shock-reversal is quicker in pediatric patients randomized to an early goal-directed fluid-sparing strategy versus usual care (SQUEEZE): study protocol for a pilot randomized controlled trial.Trials. 2016 Nov 22;17(1):556. doi: 10.1186/s13063-016-1689-2. Trials. 2016. PMID: 27876084 Free PMC article. Clinical Trial.
-
A survey of stated physician practices and beliefs on the use of steroids in pediatric fluid and/or vasoactive infusion-dependent shock.Pediatr Crit Care Med. 2013 Jun;14(5):462-6. doi: 10.1097/PCC.0b013e31828a7287. Pediatr Crit Care Med. 2013. PMID: 23628832
-
Targeted tissue perfusion versus macrocirculation-guided standard care in patients with septic shock (TARTARE-2S): study protocol and statistical analysis plan for a randomized controlled trial.Trials. 2016 Aug 2;17:384. doi: 10.1186/s13063-016-1515-x. Trials. 2016. PMID: 27484695 Free PMC article. Clinical Trial.
-
Adjunctive Steroid Therapy for Treatment of Pediatric Septic Shock.Pediatr Clin North Am. 2017 Oct;64(5):1133-1146. doi: 10.1016/j.pcl.2017.06.010. Epub 2017 Aug 18. Pediatr Clin North Am. 2017. PMID: 28941540 Review.
-
Should we abandon corticosteroids during septic shock? No.Curr Opin Crit Care. 2008 Aug;14(4):384-9. doi: 10.1097/MCC.0b013e328306a01d. Curr Opin Crit Care. 2008. PMID: 18614900 Review.
Cited by
-
Hydrocortisone Therapy in Catecholamine-Resistant Pediatric Septic Shock: A Pragmatic Analysis of Clinician Practice and Association With Outcomes.Pediatr Crit Care Med. 2017 Sep;18(9):e406-e414. doi: 10.1097/PCC.0000000000001237. Pediatr Crit Care Med. 2017. PMID: 28658197 Free PMC article.
-
Corticosteroids in Pediatric Septic Shock: A Narrative Review.J Pers Med. 2024 Dec 17;14(12):1155. doi: 10.3390/jpm14121155. J Pers Med. 2024. PMID: 39728068 Free PMC article. Review.
-
Pragmatic Pediatric Trial of Balanced Versus Normal Saline Fluid in Sepsis: The PRoMPT BOLUS Randomized Controlled Trial Pilot Feasibility Study.Acad Emerg Med. 2019 Dec;26(12):1346-1356. doi: 10.1111/acem.13815. Epub 2019 Jul 18. Acad Emerg Med. 2019. PMID: 31183919 Free PMC article.
-
Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017.Intensive Care Med. 2017 Dec;43(12):1751-1763. doi: 10.1007/s00134-017-4919-5. Epub 2017 Sep 21. Intensive Care Med. 2017. PMID: 28940011
-
A Randomized Controlled Trial of Corticosteroids in Pediatric Septic Shock: A Pilot Feasibility Study.Pediatr Crit Care Med. 2017 Jun;18(6):505-512. doi: 10.1097/PCC.0000000000001121. Pediatr Crit Care Med. 2017. PMID: 28406862 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical