Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic migraine
- PMID: 27154997
- PMCID: PMC5394468
- DOI: 10.1177/0333102416648328
Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic migraine
Abstract
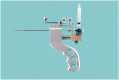
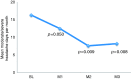
Objective The main objective of this pilot study was to investigate the safety of administering onabotulinumtoxinA towards the sphenopalatine ganglion in 10 patients with intractable chronic migraine with an open, uncontrolled design. We also collected efficacy data to provide an indication as to whether future placebo-controlled studies should be performed. Method In a prospective, open-label, uncontrolled study after one-month baseline, we performed bilateral injections of 25 IU onabotulinumtoxinA (total dose 50 IU) toward the sphenopalatine ganglion in a single outpatient session in 10 patients with intractable migraine with a follow-up of 12 weeks. The primary outcome was adverse events and the main efficacy outcome was frequency of moderate and severe headache days in month 2 post-treatment compared to baseline. Results All 10 patients experienced a total of 25 adverse events. The majority of these were different types of local discomfort in the face and jaw, and none were classified as serious. In an intention-to-treat analysis of the main efficacy outcome, a statistically significant reduction of moderate and severe headache days in baseline versus month 2 was observed (16.3 ± 6.2 days baseline versus 7.6 ± 7.6 days month 2, p = 0.009). Eight out of 10 patients experienced an at least 50% reduction of moderate and severe headache days compared to baseline. Conclusion The result warrants randomised, placebo-controlled studies to establish both safety and efficacy of this potential novel treatment of chronic migraine.
Keywords: Chronic migraine; botulinum toxin; headache; pterygopalatine ganglion; sphenopalatine ganglion.
Figures
Comment in
-
Editorial.Cephalalgia. 2017 Apr;37(4):303-304. doi: 10.1177/0333102416657131. Epub 2016 Jun 27. Cephalalgia. 2017. PMID: 27352855 No abstract available.
References
-
- Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: The Eurolight project. Eur J Neurol 2012; 19: 703–711. - PubMed
-
- Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–349. - PubMed
-
- Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 2015; 35: 478–488. - PubMed
-
- May A, Goadsby PJ. The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 1999; 19: 115–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

