Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice
- PMID: 27155103
- PMCID: PMC5308418
- DOI: 10.1016/j.yhbeh.2016.04.010
Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice
Abstract
Early life stress (ELS) increases the risk for later cognitive and emotional dysfunction. ELS is known to truncate neural development through effects on suppressing cell birth, increasing cell death, and altering neuronal morphology, effects that have been associated with behavioral profiles indicative of precocious maturation. However, how earlier silencing of growth drives accelerated behavioral maturation has remained puzzling. Here, we test the novel hypothesis that, ELS drives a switch from growth to maturation to accelerate neural and behavioral development. To test this, we used a mouse model of ELS, fragmented maternal care, and a cross-sectional dense sampling approach focusing on hippocampus and measured effects of ELS on the ontogeny of behavioral development and biomarkers of neural maturation. Consistent with previous work, ELS was associated with an earlier developmental decline in expression of markers of cell proliferation (Ki-67) and differentiation (doublecortin). However, ELS also led to a precocious arrival of Parvalbumin-positive cells, led to an earlier switch in NMDA receptor subunit expression (marker of synaptic maturity), and was associated with an earlier rise in myelin basic protein expression (key component of the myelin sheath). In addition, in a contextual fear-conditioning task, ELS accelerated the timed developmental suppression of contextual fear. Together, these data provide support for the hypothesis that ELS serves to switch neurodevelopment from processes of growth to maturation and promotes accelerated development of some forms of emotional learning.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interest.
Figures



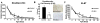
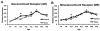
References
-
- Akers KG, Arruda-Carvalho M, Josselyn SA, Frankland PW. Ontogeny of contextual fear memory formation, specificity, and persistence in mice. Learn Mem. 2012;19:598–604. - PubMed
-
- Allsworth JE, Weitzen S, Boardman LA. Early age at menarche and allostatic load: data from the Third National Health and Nutrition Examination Survey. Ann Epidemiol. 2005;15:438–444. - PubMed
-
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

