Cold chain and virus-free chloroplast-made booster vaccine to confer immunity against different poliovirus serotypes
- PMID: 27155248
- PMCID: PMC5056803
- DOI: 10.1111/pbi.12575
Cold chain and virus-free chloroplast-made booster vaccine to confer immunity against different poliovirus serotypes
Abstract
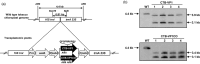
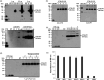
The WHO recommends complete withdrawal of oral polio vaccine (OPV) type 2 by April 2016 globally and replacing with at least one dose of inactivated poliovirus vaccine (IPV). However, high-cost, limited supply of IPV, persistent circulating vaccine-derived polioviruses transmission and need for subsequent boosters remain unresolved. To meet this critical need, a novel strategy of a low-cost cold chain-free plant-made viral protein 1 (VP1) subunit oral booster vaccine after single IPV dose is reported. Codon optimization of the VP1 gene enhanced expression by 50-fold in chloroplasts. Oral boosting of VP1 expressed in plant cells with plant-derived adjuvants after single priming with IPV significantly increased VP1-IgG1 and VP1-IgA titres when compared to lower IgG1 or negligible IgA titres with IPV injections. IgA plays a pivotal role in polio eradication because of its transmission through contaminated water or sewer systems. Neutralizing antibody titres (~3.17-10.17 log2 titre) and seropositivity (70-90%) against all three poliovirus Sabin serotypes were observed with two doses of IPV and plant-cell oral boosters but single dose of IPV resulted in poor neutralization. Lyophilized plant cells expressing VP1 stored at ambient temperature maintained efficacy and preserved antigen folding/assembly indefinitely, thereby eliminating cold chain currently required for all vaccines. Replacement of OPV with this booster vaccine and the next steps in clinical translation of FDA-approved antigens and adjuvants are discussed.
Keywords: bioencapsulation; chloroplast transformation; human infectious diseases; molecular farming; mucosal immunity; oral delivery.
© 2016 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
Although there is no financial conflict of interest to report, it is disclosed that the corresponding author is an inventor on numerous patents reporting expression of human therapeutic proteins in chloroplasts.
Figures

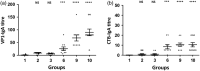
References
-
- Albarracín, R.M. , Becher, M.L. , Farran, I. , Sander, V.A. , Corigliano, M.G. , Yácono, M.L. , Pariani, S. et al. (2015) The fusion of Toxoplasma gondii SAG1 vaccine candidate to Leishmania infantum heat shock protein 83‐kDa improves expression levels in tobacco chloroplasts. Biotechnol. J. 10, 748–759. - PubMed
-
- Alexander, L.N. , Seward, J.F. , Santibanez, T.A. , Pallansch, M.A. , Kew, O.M. , Prevots, D.R. , Strebel, P.M. et al. (2004) Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA, 292, 1696–1701. - PubMed
-
- Belyakov, I.M. and Ahlers, J.D. (2009) What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 183, 6883–6892. - PubMed
-
- Bhasin, V.K. (2008) Problems with the oral polio vaccine. Nat. Med. 14, 9. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

