Development of a stable liquid formulation of live attenuated influenza vaccine
- PMID: 27155495
- PMCID: PMC4940209
- DOI: 10.1016/j.vaccine.2016.04.074
Development of a stable liquid formulation of live attenuated influenza vaccine
Abstract
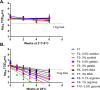
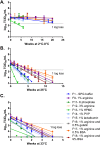
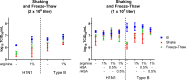
Vaccination is the most effective means of preventing influenza. However, the cost of producing annual seasonal influenza vaccines puts them out of reach for most developing countries. While live attenuated influenza vaccines are among the most efficacious and can be manufactured at low cost, they may require lyophilization to be stable enough for developing-country use, which adds a significant cost burden. The development of a liquid live attenuated seasonal influenza vaccine that is stable for around a year-the duration of an annual influenza season-would significantly improve not only the production output but also the use and accessibility of influenza vaccines in low-resource settings. In this study, potential stabilizing excipients were screened and optimized using the least stable influenza vaccine strain presently known, H1N1 (A/California/07/2009), as a model. The stability-conferring properties of the lead formulations were also tested with a Type B strain of influenza virus (B/Brisbane/60/2008). Stability was also evaluated with higher titers of influenza virus and exposure to agitation and freeze-thaw stresses to further confirm the stability of the lead formulations. Through this process, we identified a liquid formulation consisting of sucrose phosphate glutamate buffer with 1% arginine and 0.5% recombinant human serum albumin that provided storage stability of one year at 2-8°C for the influenza A and B strains tested.
Keywords: Influenza vaccine; Vaccine stabilization.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

Similar articles
-
Development of a Stable Liquid Formulation for Live Attenuated Influenza Vaccine.J Pharm Sci. 2019 Jul;108(7):2315-2322. doi: 10.1016/j.xphs.2019.02.017. Epub 2019 Feb 28. J Pharm Sci. 2019. PMID: 30826350
-
Stabilization of Live Attenuated Influenza Vaccines by Freeze Drying, Spray Drying, and Foam Drying.Pharm Res. 2016 May;33(5):1144-60. doi: 10.1007/s11095-016-1860-1. Epub 2016 Jan 27. Pharm Res. 2016. PMID: 26818839
-
Production of cell culture (MDCK) derived live attenuated influenza vaccine (LAIV) in a fully disposable platform process.Biotechnol Bioeng. 2010 Aug 15;106(6):906-17. doi: 10.1002/bit.22753. Biotechnol Bioeng. 2010. PMID: 20589670
-
Development of stable influenza vaccine powder formulations: challenges and possibilities.Pharm Res. 2008 Jun;25(6):1256-73. doi: 10.1007/s11095-008-9559-6. Pharm Res. 2008. PMID: 18338241 Free PMC article. Review.
-
The need for quadrivalent vaccine against seasonal influenza.Vaccine. 2010 Sep 7;28 Suppl 4:D45-53. doi: 10.1016/j.vaccine.2010.08.028. Vaccine. 2010. PMID: 20713260 Review.
Cited by
-
Development of a process for upscaling and production of thermotolerant Peste-des-petits ruminants vaccine.Virusdisease. 2020 Sep;31(3):357-368. doi: 10.1007/s13337-020-00608-9. Epub 2020 Jul 17. Virusdisease. 2020. PMID: 32904760 Free PMC article.
-
Thermostabilization of viruses via complex coacervation.Biomater Sci. 2020 Dec 15;8(24):7082-7092. doi: 10.1039/d0bm01433h. Biomater Sci. 2020. PMID: 33078793 Free PMC article.
-
Influenza a Neuraminidase-Based Bivalent mRNA Vaccine Induces Th1-Type Immune Response and Provides Protective Effects in Mice.Vaccines (Basel). 2024 Mar 13;12(3):300. doi: 10.3390/vaccines12030300. Vaccines (Basel). 2024. PMID: 38543934 Free PMC article.
-
Roads to advanced vaccines: influenza case study.Microb Biotechnol. 2017 Sep;10(5):1036-1040. doi: 10.1111/1751-7915.12835. Epub 2017 Aug 15. Microb Biotechnol. 2017. PMID: 28809451 Free PMC article.
-
Respiratory delivered vaccines: Current status and perspectives in rational formulation design.Acta Pharm Sin B. 2024 Dec;14(12):5132-5160. doi: 10.1016/j.apsb.2024.08.026. Epub 2024 Nov 4. Acta Pharm Sin B. 2024. PMID: 39807330 Free PMC article. Review.
References
-
- Neuzil K.M., Hohlbein C., Zhu Y. Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work, and secondary illness in families. Arch Pediatr Adolesc Med. 2002;156:986–991. - PubMed
-
- Kasowski E.J., Garten R.J., Bridges C.B. Influenza pandemic epidemiologic and virologic diversity: reminding ourselves of the possibilities. Clin Infect Dis. 2011;52(Suppl. 1):S44–S49. - PubMed
-
- Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol. 2001;260:171–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

