RecA: Regulation and Mechanism of a Molecular Search Engine
- PMID: 27156117
- PMCID: PMC4892382
- DOI: 10.1016/j.tibs.2016.04.002
RecA: Regulation and Mechanism of a Molecular Search Engine
Erratum in
-
RecA: Regulation and Mechanism of a Molecular Search Engine: (Trends in Biochemical Sciences, June 2016, Vol. 41, No. 6, 491--507).Trends Biochem Sci. 2016 Jul;41(7):646. doi: 10.1016/j.tibs.2016.05.006. Epub 2016 Jun 6. Trends Biochem Sci. 2016. PMID: 27283513 No abstract available.
Abstract
Homologous recombination maintains genomic integrity by repairing broken chromosomes. The broken chromosome is partially resected to produce single-stranded DNA (ssDNA) that is used to search for homologous double-stranded DNA (dsDNA). This homology driven 'search and rescue' is catalyzed by a class of DNA strand exchange proteins that are defined in relation to Escherichia coli RecA, which forms a filament on ssDNA. Here, we review the regulation of RecA filament assembly and the mechanism by which RecA quickly and efficiently searches for and identifies a unique homologous sequence among a vast excess of heterologous DNA. Given that RecA is the prototypic DNA strand exchange protein, its behavior affords insight into the actions of eukaryotic RAD51 orthologs and their regulators, BRCA2 and other tumor suppressors.
Keywords: Förster resonance energy transfer (FRET); RAD51; homology search; recombination; self-assembly; single molecule imaging; total internal reflection fluorescence (TIRF).
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
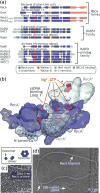
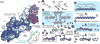


References
-
- Bianco PR, et al. DNA strand exchange proteins: A biochemical and physical comparison. Front Biosci. 1998;3:D570–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

