Design, synthesis, and biological evaluation of substrate-competitive inhibitors of C-terminal Binding Protein (CtBP)
- PMID: 27156192
- PMCID: PMC4993153
- DOI: 10.1016/j.bmc.2016.04.037
Design, synthesis, and biological evaluation of substrate-competitive inhibitors of C-terminal Binding Protein (CtBP)
Abstract
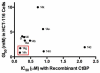
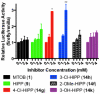
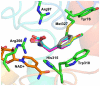
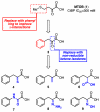
C-terminal Binding Protein (CtBP) is a transcriptional co-regulator that downregulates the expression of many tumor-suppressor genes. Utilizing a crystal structure of CtBP with its substrate 4-methylthio-2-oxobutyric acid (MTOB) and NAD(+) as a guide, we have designed, synthesized, and tested a series of small molecule inhibitors of CtBP. From our first round of compounds, we identified 2-(hydroxyimino)-3-phenylpropanoic acid as a potent CtBP inhibitor (IC50=0.24μM). A structure-activity relationship study of this compound further identified the 4-chloro- (IC50=0.18μM) and 3-chloro- (IC50=0.17μM) analogues as additional potent CtBP inhibitors. Evaluation of the hydroxyimine analogues in a short-term cell growth/viability assay showed that the 4-chloro- and 3-chloro-analogues are 2-fold and 4-fold more potent, respectively, than the MTOB control. A functional cellular assay using a CtBP-specific transcriptional readout revealed that the 4-chloro- and 3-chloro-hydroxyimine analogues were able to block CtBP transcriptional repression activity. This data suggests that substrate-competitive inhibition of CtBP dehydrogenase activity is a potential mechanism to reactivate tumor-suppressor gene expression as a therapeutic strategy for cancer.
Keywords: C-terminal Binding Protein; CtBP; Dehydrogenase; Hydroxyimine; MTOB; Oxime; Transcriptional co-repressor; Tumor suppressor gene.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

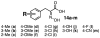



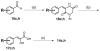
References
-
- Barroilhet L, Yang J, Hasselblatt K, Paranal RM, Ng SK, Rauh-Hain JA, Welch WR, Bradner JE, Berkowitz RS, Ng SW. Oncogene. 2013;32:3896. - PubMed
-
- Subramanian T, Chinnadurai G. FEBS Lett. 2003;540:255. - PubMed
-
- Shi Y, Sawada J.-i., Sui G, Affar EB, Whetstine JR, Lan F, Ogawa H, Po-Shan Luke M, Nakatani Y, Shi Y. Nature. 2003;422:735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

