Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan
- PMID: 27156197
- PMCID: PMC4951161
- DOI: 10.1016/j.envint.2016.03.009
Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan
Abstract
Background: There are reports of developmental and reproductive health effects associated with the widely used biocide triclosan.
Objective: Apply the Navigation Guide systematic review methodology to answer the question: Does exposure to triclosan have adverse effects on human development or reproduction?
Methods: We applied the first 3 steps of the Navigation Guide methodology: 1) Specify a study question, 2) Select the evidence, and 3) Rate quality and strength of the evidence. We developed a protocol, conducted a comprehensive search of the literature, and identified relevant studies using pre-specified criteria. We assessed the number and type of all relevant studies. We evaluated each included study for risk of bias and rated the quality and strength of the evidence for the selected outcomes. We conducted a meta-analysis on a subset of suitable data.
Results: We found 4282 potentially relevant records, and 81 records met our inclusion criteria. Of the more than 100 endpoints identified by our search, we focused our evaluation on hormone concentration outcomes, which had the largest human and non-human mammalian data set. Three human studies and 8 studies conducted in rats reported thyroxine levels as outcomes. The rat data were amenable to meta-analysis. Because only one of the human thyroxine studies quantified exposure, we did not conduct a meta-analysis of the human data. Through meta-analysis of the data for rats, we estimated for prenatal exposure a 0.09% (95% CI: -0.20, 0.02) reduction in thyroxine concentration per mg triclosan/kg-bw in fetal and young rats compared to control. For postnatal exposure we estimated a 0.31% (95% CI: -0.38, -0.23) reduction in thyroxine per mg triclosan/kg-bw, also compared to control. Overall, we found low to moderate risk of bias across the human studies and moderate to high risk of bias across the non-human studies, and assigned a "moderate/low" quality rating to the body of evidence for human thyroid hormone alterations and a "moderate" quality rating to the body of evidence for non-human thyroid hormone alterations.
Conclusion: Based on this application of the Navigation Guide systematic review methodology, we concluded that there was "sufficient" non-human evidence and "inadequate" human evidence of an association between triclosan exposure and thyroxine concentrations, and consequently, triclosan is "possibly toxic" to reproductive and developmental health. Thyroid hormone disruption is an upstream indicator of developmental toxicity. Additional endpoints may be identified as being of equal or greater concern as other data are developed or evaluated.
Keywords: Antibacterial; Personal care; Policy; Risk assessment; Soap; Thyroid.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


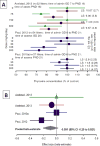
Prenatal triclosan administration and thyroxine concentration as a percentage of the control group for doses up to 300mg/kg/day
Prenatal beta-estimates for dose response and the random effects meta-analysis estimate

Postnatal triclosan administration and thyroxine concentration as a percentage of the control group for doses up to 300mg/kg/day
Postnatal beta-estimates for dose response and the random effects meta-analysis estimate
Comment in
-
More clarity needed in the Navigation Guide systematic review framework.Environ Int. 2017 May;102:74-75. doi: 10.1016/j.envint.2017.01.011. Epub 2017 Feb 17. Environ Int. 2017. PMID: 28222917 No abstract available.
-
Response to correspondence by Heather Lynch, Julie Goodman and Nancy Beck Re: "Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan".Environ Int. 2017 May;102:76-78. doi: 10.1016/j.envint.2017.02.007. Epub 2017 Feb 21. Environ Int. 2017. PMID: 28236502 Free PMC article. No abstract available.
References
-
- National Research Council. Review of EPA’s Integrated Risk Information System (IRIS) Process. Washington, DC: National Academies Press; 2014. - PubMed
-
- National Research Council. Review of the Environmental Protection Agency’s State-of-the-Science Evaluation of Nonmonotonic Dose–Response Relationships as They Apply to Endocrine Disruptors. Washington, DC: National Academies Press; 2014.
-
- Whaley PW. Systematic review and the future of evidence in chemicals policy. Réseau Environnement Santé; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

