Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation
- PMID: 27156449
- PMCID: PMC4874922
- DOI: 10.1016/j.cell.2016.04.015
Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation
Abstract
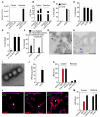
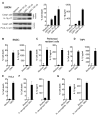
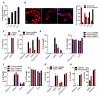
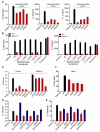
Sensing of lipopolysaccharide (LPS) in the cytosol triggers caspase-11 activation and is central to host defense against Gram-negative bacterial infections and to the pathogenesis of sepsis. Most Gram-negative bacteria that activate caspase-11, however, are not cytosolic, and the mechanism by which LPS from these bacteria gains access to caspase-11 in the cytosol remains elusive. Here, we identify outer membrane vesicles (OMVs) produced by Gram-negative bacteria as a vehicle that delivers LPS into the cytosol triggering caspase-11-dependent effector responses in vitro and in vivo. OMVs are internalized via endocytosis, and LPS is released into the cytosol from early endosomes. The use of hypovesiculating bacterial mutants, compromised in their ability to generate OMVs, reveals the importance of OMVs in mediating the cytosolic localization of LPS. Collectively, these findings demonstrate a critical role for OMVs in enabling the cytosolic entry of LPS and, consequently, caspase-11 activation during Gram-negative bacterial infections.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Inflammasomes: Intracellular detection of extracellular bacteria.Cell Res. 2016 Aug;26(8):859-60. doi: 10.1038/cr.2016.67. Epub 2016 Jun 10. Cell Res. 2016. PMID: 27283800 Free PMC article.
References
-
- Bossaller L, Chiang P-I, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VAK, Mocarski ES, Subramanian D, Green DR, Silverman N, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. The Journal of Immunology. 2012;189:5508–5512. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

