Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials
- PMID: 27156698
- PMCID: PMC4958614
- DOI: 10.1016/j.ophtha.2016.03.045
Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials
Abstract
Purpose: To describe outcomes 5 years after initiating treatment with bevacizumab or ranibizumab for neovascular age-related macular degeneration (AMD).
Design: Cohort study.
Participants: Patients enrolled in the Comparison of AMD Treatments Trials.
Methods: Patients were assigned randomly to ranibizumab or bevacizumab and to 1 of 3 dosing regimens. After 2 years, patients were released from the clinical trial protocol. At 5 years, patients were recalled for examination.
Main outcome measures: Visual acuity (VA) and morphologic retinal features.
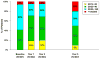
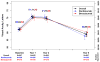
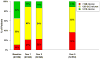
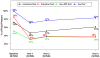
Results: Visual acuity was obtained for 647 of 914 (71%) living patients with average follow-up of 5.5 years. The mean number of examinations for AMD care after the clinical trial ended was 25.3, and the mean number of treatments was 15.4. Most patients (60%) were treated 1 time or more with a drug other than their assigned drug. At the 5-year visit, 50% of eyes had VA of 20/40 or better and 20% had VA of 20/200 or worse. Mean change in VA was -3 letters from baseline and -11 letters from 2 years. Among 467 eyes with fluorescein angiography, mean total lesion area was 12.9 mm(2), a mean of 4.8 mm(2) larger than at 2 years. Geographic atrophy was present in 213 of 515 (41%) gradable eyes and was subfoveal in 85 eyes (17%). Among 555 eyes with spectral-domain optical coherence tomography, 83% had fluid (61% intraretinal, 38% subretinal, and 36% sub-retinal pigment epithelium). Mean foveal total thickness was 278 μm, a decrease of 182 μm from baseline and 20 μm from 2 years. The retina was abnormally thin (<120 μm) in 36% of eyes. Between 2 and 5 years, the group originally assigned to ranibizumab for 2 years lost more VA than the bevacizumab group (-4 letters; P = 0.008). Otherwise, there were no statistically significant differences in VA or morphologic outcomes between drug or regimen groups.
Conclusions: Vision gains during the first 2 years were not maintained at 5 years. However, 50% of eyes had VA of 20/40 or better, confirming anti-vascular endothelial growth factor therapy as a major long-term therapeutic advance for neovascular AMD.
Copyright © 2016 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Disclosure: Dr. Maguire reports personal fees from Roche/Genentech; Dr. Ying reports personal fees from Janssen R & D and Chengdu Kanghong Biotech Co; Dr. Jaffe reports personal fees from Alcon/Novartis, Neurotech, and Roche/Genentech; Dr. Toth grants from Genentech, Inc., personal fees from Alcon, Inc. and Thrombogenics, non-financial support from Bioptigen, and a patent unlicensed issued to Duke University.
Figures



Comment in
-
Re: Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group, et al.: Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials (Ophthalmology 2016;123:1751-1761).Ophthalmology. 2017 Mar;124(3):e31-e32. doi: 10.1016/j.ophtha.2016.05.054. Ophthalmology. 2017. PMID: 28219517 No abstract available.
-
Reply.Ophthalmology. 2017 Mar;124(3):e33. doi: 10.1016/j.ophtha.2016.06.046. Ophthalmology. 2017. PMID: 28219518 No abstract available.
References
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. - PubMed
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. - PubMed
-
- Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. - PubMed
-
- Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128:1273–1279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

