Immune-mediated reactions to vancomycin: A systematic case review and analysis
- PMID: 27156746
- PMCID: PMC4946960
- DOI: 10.1016/j.anai.2016.03.030
Immune-mediated reactions to vancomycin: A systematic case review and analysis
Abstract
Background: Vancomycin is a broad-spectrum antibiotic whose use may be limited by adverse drug reactions (ADRs). Although vancomycin toxic effects are known, there are limited data on vancomycin hypersensitivity reactions (HSRs).
Objective: To understand the most commonly reported vancomycin HSRs through systematic case review.
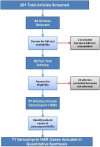
Methods: We performed a literature search for English-language case reports and series from 1982 through 2015 (last search July 31, 2015) on Ovid MEDLINE and PubMed. The search included the subject heading vancomycin with the subheading adverse effects and separate text searches for vancomycin with a list of specified HSRs. References of identified articles were reviewed to find additional articles. Clinical data were collected and summarized.
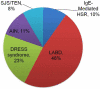
Results: Of 201 identified articles, 84 were screened and 57 fully assessed; these 57 articles contained 71 vancomycin HSR cases that were included in analysis. Vancomycin HSRs were immediate (anaphylaxis, n = 7) and nonimmediate (n = 64). Nonimmediate HSRs included linear IgA bullous dermatosis (LABD, n = 34), drug rash eosinophilia and systemic symptoms (DRESS) syndrome (n = 16), acute interstitial nephritis (AIN, n = 8), and Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN, n = 6). Median times of vancomycin therapy before HSR onset was 7 days (interquartile range [IQR], 4-10 days) for LABD, 9 days (IQR, 9-22 days) for SJS/TEN, 21 days (IQR, 17-28 days) for DRESS syndrome, and 26 days (IQR, 7-29 days) for AIN. Overall, 11 patients (16%) died, and 4 (6%) had deaths attributed to the HSR.
Conclusion: Vancomycin causes a variety of HSRs; the most commonly identified were nonimmediate HSRs, with LABD being most frequent. We observed a high frequency of HSR mortality. Further data are needed to understand the frequency and severity of vancomycin HSRs.
Copyright © 2016 American College of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi:10.2146/ajhp080434. - PubMed
-
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi:10.2146/ajhp120568. - PubMed
-
- Bruniera R. The use of vancomycin with its therapeutic and adverse effects : a review. 2015. pp. 694–700. - PubMed
-
- Wynn M, Dalovisio JR, Tice AD, Jiang X. Evaluation of the efficacy and safety of outpatient parenteral antimicrobial therapy for infections with methicillin-sensitive Staphylococcus aureus. South Med J. 2005;98(6):590–595. doi:10.1097/01.SMJ.0000145300.28736.BB. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

