Ratiometric QD-FRET Sensing of Aqueous H2S in Vitro
- PMID: 27156947
- PMCID: PMC4899257
- DOI: 10.1021/acs.analchem.6b01310
Ratiometric QD-FRET Sensing of Aqueous H2S in Vitro
Abstract
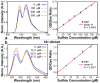
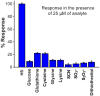
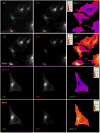
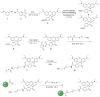
We report a platform for the ratiometric fluorescent sensing of endogenously generated gaseous transmitter H2S in its aqueous form (bisulfide or hydrogen sulfide anion) based on the alteration of Förster resonance energy transfer from an emissive semiconductor quantum dot (QD) donor to a dithiol-linked organic dye acceptor. The disulfide bridge between the two chromophores is cleaved upon exposure to bisulfide, resulting in termination of FRET as the dye diffuses away from the QD. This results in enhanced QD emission and dye quenching. The resulting ratiometric response can be correlated quantitatively to the concentration of bisulfide and was found to have a detection limit as low as 1.36 ± 0.03 μM. The potential for use in biological applications was demonstrated by measuring the response of the QD-based FRET sensor microinjected into live HeLa cells upon extracellular exposure to bisulfide. The methodology used here is built upon a highly multifunctional platform that offers numerous advantages, such as low detection limit, enhanced photochemical stability, and sensing ability within a biological milieu.
Figures
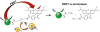
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

