Loss of the 14-3-3σ is essential for LASP1-mediated colorectal cancer progression via activating PI3K/AKT signaling pathway
- PMID: 27156963
- PMCID: PMC4860602
- DOI: 10.1038/srep25631
Loss of the 14-3-3σ is essential for LASP1-mediated colorectal cancer progression via activating PI3K/AKT signaling pathway
Abstract
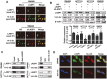
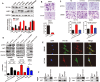
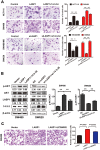
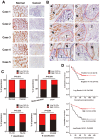
LIM and SH3 protein 1 (LASP1) can promote colorectal cancer (CRC) progression and metastasis, but the direct evidence that elucidates the molecular mechanism remains unclear. Here, our proteomic data showed that LASP1 interacted with 14-3-3σ and decreased the expression of 14-3-3σ in CRC. Deletion of 14-3-3σ was required for LASP1-mediated CRC cell aggressiveness. In vitro gain- and loss-of-function assays showed that 14-3-3σ suppressed the ability of cell migration and decreased the phosphorylation of AKT in CRC cells. We further observed clearly co-localization between AKT and 14-3-3σ in CRC cells. Treatment of PI3K inhibitor LY294002 markedly prevented phosphorylation of AKT and subsequently counteract aggressive phenotype mediated by siRNA of 14-3-3σ. Clinically, 14-3-3σ is frequently down-regulated in CRC tissues. Down-regulation of 14-3-3σ is associated with tumor progression and poor prognosis of patients with CRC. Multivariate analysis confirmed low expression of 14-3-3σ as an independent prognostic factor for CRC. A combination of low 14-3-3σ and high LASP1 expression shows a worse trend with overall survival of CRC patients. Our research paves the path to future investigation of the LASP1-14-3-3σ axis as a target for novel anticancer therapies of advanced CRC.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
COPS5 and LASP1 synergistically interact to downregulate 14-3-3σ expression and promote colorectal cancer progression via activating PI3K/AKT pathway.Int J Cancer. 2018 May 1;142(9):1853-1864. doi: 10.1002/ijc.31206. Epub 2017 Dec 27. Int J Cancer. 2018. PMID: 29226323
-
ECHS1, an interacting protein of LASP1, induces sphingolipid-metabolism imbalance to promote colorectal cancer progression by regulating ceramide glycosylation.Cell Death Dis. 2021 Oct 6;12(10):911. doi: 10.1038/s41419-021-04213-6. Cell Death Dis. 2021. PMID: 34615856 Free PMC article.
-
LIM and SH3 protein 1 induces glioma growth and invasion through PI3K/AKT signaling and epithelial-mesenchymal transition.Biomed Pharmacother. 2019 Aug;116:109013. doi: 10.1016/j.biopha.2019.109013. Epub 2019 May 27. Biomed Pharmacother. 2019. PMID: 31146105
-
LASP1 in Cellular Signaling and Gene Expression: More than Just a Cytoskeletal Regulator.Cells. 2022 Nov 29;11(23):3817. doi: 10.3390/cells11233817. Cells. 2022. PMID: 36497077 Free PMC article. Review.
-
LASP1 in Tumor and Tumor Microenvironment.Curr Mol Med. 2017;17(8):541-548. doi: 10.2174/1566524018666180222115103. Curr Mol Med. 2017. PMID: 29473505 Review.
Cited by
-
Stratifin (SFN) Regulates Cervical Cancer Cell Proliferation, Apoptosis, and Cytoskeletal Remodeling and Metastasis Progression Through LIMK2/Cofilin Signaling.Mol Biotechnol. 2024 Nov;66(11):3369-3381. doi: 10.1007/s12033-023-00946-1. Epub 2023 Nov 9. Mol Biotechnol. 2024. PMID: 37946061 Free PMC article.
-
LIM and SH3 protein 1 regulates cell growth and chemosensitivity of human glioblastoma via the PI3K/AKT pathway.BMC Cancer. 2018 Jul 6;18(1):722. doi: 10.1186/s12885-018-4649-2. BMC Cancer. 2018. PMID: 29980193 Free PMC article.
-
Cysteine-rich intestinal protein 1 suppresses apoptosis and chemosensitivity to 5-fluorouracil in colorectal cancer through ubiquitin-mediated Fas degradation.J Exp Clin Cancer Res. 2019 Mar 8;38(1):120. doi: 10.1186/s13046-019-1117-z. J Exp Clin Cancer Res. 2019. PMID: 30850009 Free PMC article.
-
Network pharmacology and experimental analysis to reveal the mechanism of Dan-Shen-Yin against endothelial to mesenchymal transition in atherosclerosis.Front Pharmacol. 2022 Aug 24;13:946193. doi: 10.3389/fphar.2022.946193. eCollection 2022. Front Pharmacol. 2022. PMID: 36091823 Free PMC article.
-
MiR-133a acts as a tumor suppressor in lung cancer progression by regulating the LASP1 and TGF-β/Smad3 signaling pathway.Thorac Cancer. 2020 Dec;11(12):3473-3481. doi: 10.1111/1759-7714.13678. Epub 2020 Oct 19. Thorac Cancer. 2020. PMID: 33074595 Free PMC article.
References
-
- Grunewald T. G. et al.. Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res 312, 974–982 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

