Tumour-Derived Glutamate: Linking Aberrant Cancer Cell Metabolism to Peripheral Sensory Pain Pathways
- PMID: 27157265
- PMCID: PMC5543678
- DOI: 10.2174/1570159X14666160509123042
Tumour-Derived Glutamate: Linking Aberrant Cancer Cell Metabolism to Peripheral Sensory Pain Pathways
Abstract
Background: Chronic pain is a major symptom that develops in cancer patients, most commonly emerging during advanced stages of the disease. The nature of cancer-induced pain is complex, and the efficacy of current therapeutic interventions is restricted by the dose-limiting sideeffects that accompany common centrally targeted analgesics.
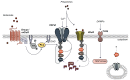
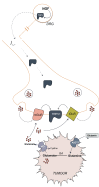
Methods: This review focuses on how up-regulated glutamate production and export by the tumour converge at peripheral afferent nerve terminals to transmit nociceptive signals through the transient receptor cation channel, TRPV1, thereby initiating central sensitization in response to peripheral disease-mediated stimuli.
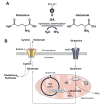
Results: Cancer cells undergo numerous metabolic changes that include increased glutamine catabolism and over-expression of enzymes involved in glutaminolysis, including glutaminase. This mitochondrial enzyme mediates glutaminolysis, producing large pools of intracellular glutamate. Upregulation of the plasma membrane cystine/glutamate antiporter, system xc -, promotes aberrant glutamate release from cancer cells. Increased levels of extracellular glutamate have been associated with the progression of cancer-induced pain and we discuss how this can be mediated by activation of TRPV1.
Conclusion: With a growing population of patients receiving inadequate treatment for intractable pain, new targets need to be considered to better address this largely unmet clinical need for improving their quality of life. A better understanding of the mechanisms that underlie the unique qualities of cancer pain will help to identify novel targets that are able to limit the initiation of pain from a peripheral source-the tumour.
Keywords: Cancer pain; TRPV1; glutamate; glutaminase; system xc.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures
Similar articles
-
Functional effects of TrkA inhibition on system xC--mediated glutamate release and cancer-induced bone pain.Mol Pain. 2018 Jan-Dec;14:1744806918776467. doi: 10.1177/1744806918776467. Mol Pain. 2018. PMID: 29761734 Free PMC article.
-
The cystine/glutamate antiporter system xc- drives breast tumor cell glutamate release and cancer-induced bone pain.Pain. 2016 Nov;157(11):2605-2616. doi: 10.1097/j.pain.0000000000000681. Pain. 2016. PMID: 27482630 Free PMC article.
-
Identifying spinal sensory pathways activated by noxious esophageal acid.Neurogastroenterol Motil. 2013 Oct;25(10):e660-8. doi: 10.1111/nmo.12180. Epub 2013 Jul 12. Neurogastroenterol Motil. 2013. PMID: 23848546
-
Feedback mechanisms in the regulation of intracellular calcium ([Ca2+]i) in the peripheral nociceptive system: role of TRPV-1 and pain related receptors.Cell Calcium. 2008 Mar;43(3):215-27. doi: 10.1016/j.ceca.2007.05.019. Epub 2007 Jul 27. Cell Calcium. 2008. PMID: 17673288 Review.
-
Modulation of sensory input to the spinal cord by presynaptic ionotropic glutamate receptors.Arch Ital Biol. 2005 May;143(2):103-12. Arch Ital Biol. 2005. PMID: 16106991 Review.
Cited by
-
nNav1.5 expression is associated with glutamate level in breast cancer cells.Biol Res. 2022 Apr 29;55(1):18. doi: 10.1186/s40659-022-00387-1. Biol Res. 2022. PMID: 35488278 Free PMC article.
-
Inhibiting STAT3 in a murine model of human breast cancer-induced bone pain delays the onset of nociception.Mol Pain. 2019 Jan-Dec;15:1744806918823477. doi: 10.1177/1744806918823477. Mol Pain. 2019. PMID: 30799695 Free PMC article.
-
xCT knockdown in human breast cancer cells delays onset of cancer-induced bone pain.Mol Pain. 2019 Jan-Dec;15:1744806918822185. doi: 10.1177/1744806918822185. Mol Pain. 2019. PMID: 30799686 Free PMC article.
-
Cell death in head and neck cancer pathogenesis and treatment.Cell Death Dis. 2021 Feb 18;12(2):192. doi: 10.1038/s41419-021-03474-5. Cell Death Dis. 2021. PMID: 33602906 Free PMC article. Review.
-
Current State of Melanoma Therapy and Next Steps: Battling Therapeutic Resistance.Cancers (Basel). 2024 Apr 19;16(8):1571. doi: 10.3390/cancers16081571. Cancers (Basel). 2024. PMID: 38672652 Free PMC article. Review.
References
-
- Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. [http://dx.doi.org/10.1016/j.cell.2009.09.028]. [PMID: 19837031]. - PMC - PubMed
-
- Delmas P., Hao J., Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 2011;12(3):139–153. [http://dx.doi.org/10.1038/ nrn2993]. [PMID: 21304548]. - PubMed
-
- Broman J., Anderson S., Ottersen O.P. Enrichment of glutamate-like immunoreactivity in primary afferent terminals throughout the spinal cord dorsal horn. Eur. J. Neurosci. 1993;5(8):1050–1061. [http://dx.doi.org/10.1111/j.1460-9568.1993.tb00958.x]. [PMID: 7904222]. - PubMed
-
- De Biasi S., Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc. Natl. Acad. Sci. USA. 1988;85(20):7820–7824. [http://dx. doi.org/10.1073/pnas.85.20.7820]. [PMID: 2459717]. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
