Long-term treatment with aldosterone slows the progression of age-related hearing loss
- PMID: 27157488
- PMCID: PMC7416673
- DOI: 10.1016/j.heares.2016.05.001
Long-term treatment with aldosterone slows the progression of age-related hearing loss
Abstract
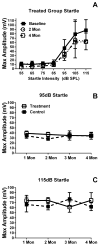
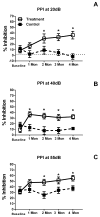
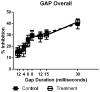
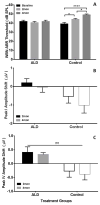
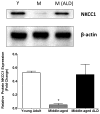
Age-related hearing loss (ARHL), clinically referred to as presbycusis, is one of the three most prevalent chronic medical conditions of our elderly, with the majority of persons over the age of 60 suffering from some degree of ARHL. The progressive loss of auditory sensitivity and perceptual capability results in significant declines in workplace productivity, quality of life, cognition and abilities to communicate effectively. Aldosterone is a mineralocorticoid hormone produced in the adrenal glands and plays a role in the maintenance of key ion pumps, including the Na-K(+)-Cl co-transporter 1 or NKCC1, which is involved in homeostatic maintenance of the endocochlear potential. Previously we reported that aldosterone (1 μM) increases NKCC1 protein expression in vitro and that this up-regulation of NKCC1 was not dose-dependent (dosing range from 1 nM to 100 μM). In the current study we measured behavioral and electrophysiological hearing function in middle-aged mice following long-term systemic treatment with aldosterone. We also confirmed that blood pressure remained stable during treatment and that NKCC1 protein expression was upregulated. Pre-pulse inhibition of the acoustic startle response was used as a functional measure of hearing, and the auditory brainstem response was used as an objective measure of peripheral sensitivity. Long-term treatment with aldosterone improved both behavioral and physiological measures of hearing (ABR thresholds). These results are the first to demonstrate a protective effect of aldosterone on age-related hearing loss and pave the way for translational drug development, using aldosterone as a key component to prevent or slow down the progression of ARHL.
Keywords: Aging; Aldosterone; Drug development; Hearing loss; Startle reflex.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Age-related hearing loss: prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons.Aging (Albany NY). 2016 Sep 23;8(9):2081-2099. doi: 10.18632/aging.101045. Aging (Albany NY). 2016. PMID: 27667674 Free PMC article.
-
L-Ergothioneine slows the progression of age-related hearing loss in CBA/CaJ mice.Hear Res. 2024 May;446:109004. doi: 10.1016/j.heares.2024.109004. Epub 2024 Apr 2. Hear Res. 2024. PMID: 38608332 Free PMC article.
-
Low-frequency tone pips elicit exaggerated startle reflexes in C57BL/6J mice with hearing loss.J Assoc Res Otolaryngol. 2003 Dec;4(4):495-504. doi: 10.1007/s10162-002-3046-2. Epub 2003 Jun 6. J Assoc Res Otolaryngol. 2003. PMID: 12784135 Free PMC article.
-
Understanding hormone and hormone therapies' impact on the auditory system.J Neurosci Res. 2020 Sep;98(9):1721-1730. doi: 10.1002/jnr.24588. Epub 2020 Feb 5. J Neurosci Res. 2020. PMID: 32026519 Review.
-
Genetic influences on susceptibility of the auditory system to aging and environmental factors.Scand Audiol Suppl. 1992;36:1-39. Scand Audiol Suppl. 1992. PMID: 1488615 Review.
Cited by
-
Age-related hearing loss: prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons.Aging (Albany NY). 2016 Sep 23;8(9):2081-2099. doi: 10.18632/aging.101045. Aging (Albany NY). 2016. PMID: 27667674 Free PMC article.
-
Association between a High-Potassium Diet and Hearing Thresholds in the Korean Adult Population.Sci Rep. 2019 Jul 4;9(1):9694. doi: 10.1038/s41598-019-45930-5. Sci Rep. 2019. PMID: 31273228 Free PMC article.
-
Age-related changes of auditory sensitivity across the life span of CBA/CaJ mice.Hear Res. 2024 Jan;441:108921. doi: 10.1016/j.heares.2023.108921. Epub 2023 Nov 22. Hear Res. 2024. PMID: 38042127 Free PMC article.
-
Aldosterone up-regulates voltage-gated potassium currents and NKCC1 protein membrane fractions.Sci Rep. 2020 Sep 24;10(1):15604. doi: 10.1038/s41598-020-72450-4. Sci Rep. 2020. PMID: 32973172 Free PMC article.
-
Machine learning, waveform preprocessing and feature extraction methods for classification of acoustic startle waveforms.MethodsX. 2020 Dec 1;8:101166. doi: 10.1016/j.mex.2020.101166. eCollection 2021. MethodsX. 2020. PMID: 33354518 Free PMC article.
References
-
- Ding B, Frisina RD, Zhu X, Frisina DR, Walton JP. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; 2012. Aldosterone increases the protein expression of Na-K-Cl cotransporter (NKCC1) via an ubiquitination mechanism. Program No. 367.10. Online.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

